13.3
Impact Factor
Theranostics 2019; 9(11):3134-3149. doi:10.7150/thno.33539 This issue Cite
Review
Metal-Organic Framework Nanoparticle-Based Biomineralization: A New Strategy toward Cancer Treatment
1. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics & Center for Molecular Imaging and Translational Medicine, School of Public Health Xiamen, Xiamen University, Xiamen 361102, China
2. State Key Laboratory of Physical Chemistry of Solid Surfaces & The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
3. Laboratory of Molecular Imaging and Nanomedicine, National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), Bethesda, MD 20892, USA
# These authors contributed equally to this work.
Received 2019-1-27; Accepted 2019-3-20; Published 2019-5-18
Abstract
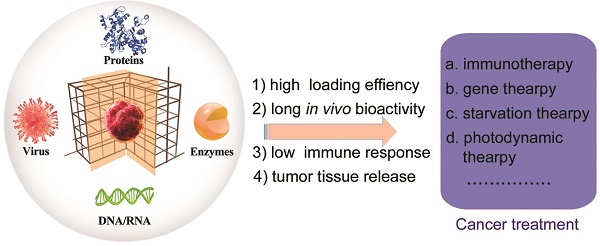
Cancer treatment using functional proteins, DNA/RNA, or complex bio-entities is important in both preclinical and clinical studies. With the help of nano-delivery systems, these biomacromolecules can enrich cancer tissues to match the clinical requirements. Biomineralization via a self-assembly process has been widely applied to provide biomacromolecules exoskeletal-like protection for immune shielding and preservation of bioactivity. Advanced metal-organic framework nanoparticles (MOFs) are excellent supporting matrices due to the low toxicity of polycarboxylic acids and metals, high encapsulation efficiency, and moderate synthetic conditions. In this review, we study MOFs-based biomineralization for cancer treatment and summarize the unique properties of MOF hybrids. We also evaluate the outlook of potential cancer treatment applications for MOFs-based biomineralization. This strategy likely opens new research orientations for cancer theranostics.
Keywords: metal-organic framework, biomineralization, cancer treatment, theranostics
1. Introduction
Cancer is an urgent global public problem and the focus of significant worldwide research [1-3]. Cancer treatments include low-molecular-weight drugs, gene therapy via DNA/RNA [4], immunotherapy via antibodies and antigens [5], and biotherapy via proteins and oncolytic viruses [6]. However, these biological approaches are limited by the complicated tumor microenvironment, so the delivery systems were studied for the application of the biomacromolecules. These traditional delivery systems were designed to load the biomacromolecules on the surface of the nanomaterials, or coated inner the nanomaterials while the self-assembly of the polymer or amphipathic molecule. And, with help of the target modification, the biomacromolecules could gather in the function tissues/cells.
Recently, many researchers have focused on metal-organic frameworks (MOFs), which are novel porous materials [7-11]. MOFs are constructed by the coordination of metal cations or clusters and organic bridging ligands. They offer a tunable design and a network structure with controlled chemical functionality, high crystallinity, and good porosity. Due to their unique structures and properties, MOFs have been applied in gas storage and separations [12], catalysis [13, 14], energy [15, 16], and sensing [17, 18].
Nanomedicines were designed to overcome biological barriers and selectively target tissues. They are efficient therapeutics [19]. MOFs can be tailored for specific biomedical applications [20-23]. MOFs are promising platforms for molecular imaging or drug delivery because of their porosity, tunable design, and low toxicity [24-28]. Furthermore, MOFs were also good delivery platforms for the biomacromolecules and the loading strategies of biomacromolecules can be categorized in four ways: (i) the biomacromolecule was adsorbed on the surface of MOFs due to the physical absorption [29-30]; (ii) the biomacromolecule was conjugated on the surface through the chemical coupling with the organic bridging ligands or the chelation reactions with the chelation reactions [31-33]; (iii) the biomacromolecule was infiltrated into the pore taking advantage of the mesoporous nanostructure [34-37]; (iv) the biomacromolecule was encapsulated within the MOFs networks during the self-assembly reaction of the mixed solution containing metal cations, organic bridging ligands and the biomacromolecules [38-40].
These MOF biocomposites obtained by surface coating or bio-conjugation enabled the biomacromolecule in the outermost surface, which the MOFs mostly serve as the carrier but leads to insufficient protection of biomacromolecules. While the biomacromolecules loaded on the pore networks of MOFs could protect the biomacromolecules from the external environment, and yet the infiltration strategy was dependent on pore size of the MOFs, in which the pore size must exceed the biomacromolecule.
During the self-assembly (biomineralization), the activity of the biomolecules or living organisms are retained and even improved in some cases [41-44]. The diversity of the metal-connecting points and organic-bridging ligands as well as straightforward self-assembly makes MOFs excellent supporting matrices for the immobilization of DNA, enzymes, peptides, proteins and living organisms (e.g., viruses and cells) (Figure 1) [22]. Furthermore, these surface-coated matrices of MOFs help the biomolecules or living organisms with prolonged bio-activity to enhance their stability under physiological conditions. MOFs-based biomineralization has been applied for storage, transport, treatment, and sensing. Cargo includes biological catalysts, biomolecules, or living organisms [41].
In this review, we summarize the application of MOFs-based biomineralization in cancer treatment (Figure 1). We discuss potential obstacles for practical use including toxicity arising from unintended interactions of MOFs with healthy organisms and the relevant implications for rational design. We also detail relevant insights into future applications of MOFs and how nano-bio interactions will be key to the safe design of MOFs as a platform for cancer treatment.
2. MOFs-based biomineralization of proteins
Protein-based pharmaceuticals (Pps), a kind of high-molecular weight therapeutic substance (>1kDa), have emerged as dominant tools (including cytokines, therapeutic antibody, protease and protein vaccines) in the treatment of various cancer [45]. Compared with traditional small molecule chemotherapeutics, Pps have tunable properties, increased therapeutic efficacy and reduced systemic toxicity [46-50]. Since rituximab was approval for lymphoma target therapy in 1997, therapeutic proteins have become a fast-growing category among cancer therapeutic drugs [51-62]. Data from various La Merie financial reports indicate that total sales of Pps in 2017 reached $188 billion, and the rapid growth will continue over the next several years.
MOFs-based biomineralization of proteins, enzymes, DNA/RNA and virus, and their applications in cancer treatment.
Summary of preparations and applications of the protein-biomineralized MOFs.
| MOF | Protein | Size | Application | Ligand | Metal ion | Temperature | Time | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZIF-8 | BSA, HSA, OVA, HRP, insulin, hemoglobin, lysozyme | 1-5 μm | Bioprocessing or delivery | 2-methylimidazole (BSA) | Zn2+ (BSA) | Rt (BSA) | 12 h (BSA) | [22] |
| ZIF-90 | catalase | ~1.5 μm | Biocatalysis | Dithiodiglycolic acid ( disulfide ) | Mn2+ | 150 ℃ | 24 h | [74] |
| ZIF-8 | GOx | >1 μm | Biocatalysis | 2-methylimidazole | Zn2+ | 4 ℃ | 12 h | [127] |
| NU-1003 | organophosphorus acid anhydrolase (OPAA) | 300 nm (minimum size) | Biocatalysis | benzoicacid | Zr4+ | 80 ℃ | 1 h | [145] |
| ZIF-8 | urease | 120-500 nm | Bioprocessing | 2-methylimidazole | Zn2+ | Rt | Over-night | [146] |
| NPCN-333 | tyrosinase | 100 nm | Biocatalysis and cancer therapy | 4,4',4''-s-triazine-2,4,6-triyl-tribenzoic acid (TATB) | Al3+ | 95 ℃ | 24 h | [147] |
| ZIF-8 | insulin/GOx, GOx/HRP or β-Gal/GOx/HRP | ~ 500 nm | Biocatalysis | 2-methylimid azole (insulin) | Zn2+ (insulin) | / | Over-night | [137, 143] |
| ZIF-8 | BSA (DOX) | 70-110 nm | Cancer drug delivery | 2-methylimidazole | Zn2+ | / | 10 min | [148] |
| ZIF-8 | OVA | ~ 200 nm | Cancer immunotherapy | 2-methylimidazole | Zn2+ | Rt | 10 min | [109] |
| ZIF-8 | BSA | 53-153 nm | Bioprocessing for cancer treatment | 2-methylimidazole | Zn2+ | 30 ℃ | 20 min | [75] |
| ZIF-8 | BSA, cytochrome c or gelonin | < 100 nm | Bioprocessing for cancer treatment | 2-methylimidazole (BSA) | Zn2+ (BSA) | / | 50 min (BSA) | [88] |
The predominance of protein-based biopharmaceuticals is likely to remain among the anticancer drugs in the foreseeable future. For example, monoclonal antibody is one of the most successful anticancer biopharmaceuticals, which has been developed and applied to treat various cancers (e.g., breast cancer, colorectal cancer, lung cancer, et al.). As the fastest growing segment of the biopharmaceutical market, protein-based biopharmaceuticals have significantly extended the lives of many cancer patients. However, proteins are xenobiotics that can elicit adverse immune reactions [6], and may lose their biological activity or even be degraded at low pH and near proteolytic enzymes in the tumor microenvironment [63-65]. With the help of advanced delivery nanosystems, bioactive proteins can be efficiently delivered to the tumor tissues/cells for targeted anticancer theranostics.
The encapsulation of protein in MOF architectures provides a novel strategy for its efficient loading without concerning the size of the biomolecules [41, 66-67]. The co-precipitation during the self-assembly procedure of the MOFs precursor solution containing the protein is a one-pot embedding. Furthermore, the addition of the protein also promotes the rapid growth of MOFs. To date, the study of the MOFs biomineralized proteins has attracted the researchers a wide range of interests, and the applicability of this strategy has demonstrated toward different proteins and MOFs (Table 1).
Recently, Liang et al. reported a simple approach to encapsulate bovine serum albumin (BSA) and horseradish peroxidase (HRP) into MOFs by de novo assembly [11]. This is especially useful for biomineralization through the crystal growth of the zeolitic imidazolate framework-8 (ZIF-8) using Zn(II) ion and 2-methylimidazole (2-MIM). The controlled crystal formation enabled each BSA to be encapsulated with ~22 Zn(II) ion and ~31 2-MIM; the protein encapsulation efficiencies were 82% to even near 100% for different proteins. More importantly, the encapsulated proteins retained bioactivity at elevated temperatures, low pH, and organic solvents, leading to improved outcomes.
In another example, Feng et al reported the preparation of Antibody@MOFs by a facile biomineralization procedure [68]. The in vitro self-assembly reaction could protect the antibodies in the framework, and the antibodies could release within 10 s. After encapsulated by the ZIF-90 or ZIF-8, the Human immunoglobulin G (IgG) polyclonal antibody (H-IgG) and Goat anti BSA IgG polyclonal antibody (G-IgG) could show few aggregations in the harsh condition including high temperature (75 °C), methanol or acetone, freeze-thawing cycles (-80 °C~37 °C) and high pressure (20 Mpa). Furthermore, the adalimumab (Ada) was biomineralized by ZIF-90 or ZIF-8 with high encapsulation efficiencies, and high recover efficiencies. After treated with 5 °C heat or 5 days storage plus 10 freeze-thawing cycles, the protected Ada could recover high bioactivities.
The good protection effect was quite an advantage for the protein@MOFs to adapt the complex tumor environment, and showed potential application to the cancer treatment. The protein@MOFs must possess good biocompatibility and low immunological risks [69-73]. In addition, most of the early MOFs-based biomineralization of protein used micron-sized carriers display limitations in vivo applications (Table 1) [22, 74]. With the development of the MOF preparation, the size, Zeta potential, and biocompatibility of some protein-encapsulated MOFs could match the needs of nanomedicine. In 2018, Chen et al. synthesized BSA@ZIF-8 NPs with a size of 92 ± 7.9 nm. The encapsulation efficiency was 93%, and the BSA loading capacity was ∼52.2 μg in 1 mg BSA@ZIF-8 NPs—these were useful for intracellular studies [75]. The BSA delivery system was also extended to other proteins and even multiple proteins in one single ZIF-8 NPs for co-delivery with high loading capacities; only the size changed. This might be due to the different pre-nucleated clusters surrounding the proteins.
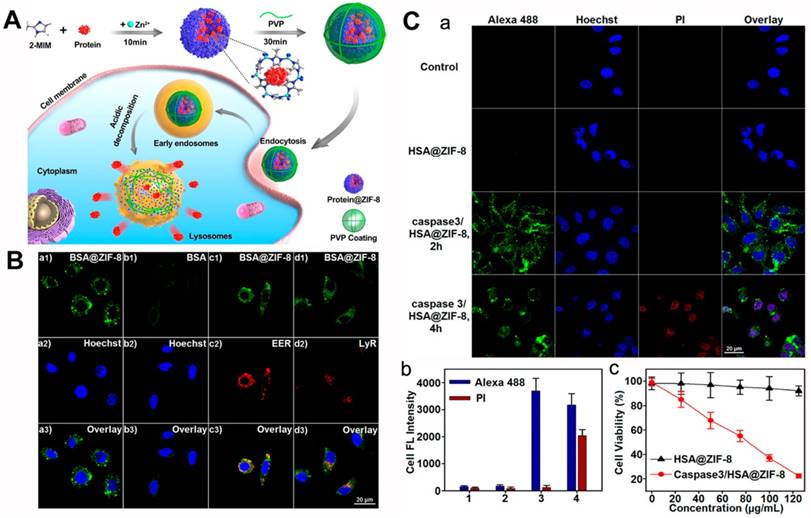
The protein-encapsulated ZIF-8 NPs was further coated with a layer of polyvinylpyrrolidone (PVP) to enhance its stability in cell media (Figure 2). The BSA@MOF hybrid could decompose in acidic buffer (pH = 5.5), and the BSA was released within 0.5 h and completed after 2 h. Next, the fluorescein (FITC)-labeled BSA was incubated in the ZIF-8 NPs, and the resulting MOF hybrid offered much higher FITC fluorescence than the pure BSA in the HeLa cells, implying that the PVP-coated MOF hybrid improved the delivery efficiency of protein. In addition, the cell fluorescence study of the early endosomes and lysosomes indicated that the endocytic pathway accounted for the MOF hybrid. Notably, the ZIF-8 NPs could be decomposed in the weakly acid environment of early endosomes and lysosomes. The encapsulated protein was then released into the cytoplasm. The development of the MOF hybrid could enable the proteins delivered to the live cell and escaped from the endo-lysosomes to avoid degradation/denaturation. Thus, the biomineralized MOF designed with this rationale holds great potential for cancer theranostics.
(1) Therapeutic proteins
Nowadays, many therapeutic proteins were applied in the cancer therapy. However, the systemic delivery of therapeutic proteins to target cancer sites is impeded by many factors including rapid degradation and systemic elimination of 'naked' proteins in biological systems. A variety of delivery nanosystems have been invented to deliver therapeutic proteins to target cancer tissues [76-79]. For example, the tumor necrosis factor-related apoptosis inducing ligand (TRAIL) could induce the apoptosis of cancer cells sparing normal tissue [80-83]. To effective delivery the TRAIL ligands, Lin et al applied a micellar hybrid nanoparticle to carry TRAIL (IPN@TRAIL) [6]. The IPN@TRAIL could localize to the tumor tissues, and the TRAIL therapeutic efficiency enhanced by TRAIL nano-vectorization.
(A) Illustration of MOFs-based biomineralization and surface modification, and the intracellular delivery of MOF hybrid. (B) The cell fluorescence of BSA@MOF hybrid (a), FITC-BSA (b), BSA@MOF hybrid and early endosomes localized EER (c), BSA@MOF hybrid and lysosome localized LyR (d). (C) The study of the caspase 3/HSA@ MOF hybrid: (a) The cell fluorescence of HSA and caspase 3 co-encapsulated MOF hybrid. (b) The fluorescence intensity of control cells (1), HSA@MOF hybrid (2), caspase 3/HSA@ MOF hybrid 2 h (3) and 4 (4). (c)The cell viability after treatment with different MOF hybrids [75]. Copyright 2018 American Chemical Society.
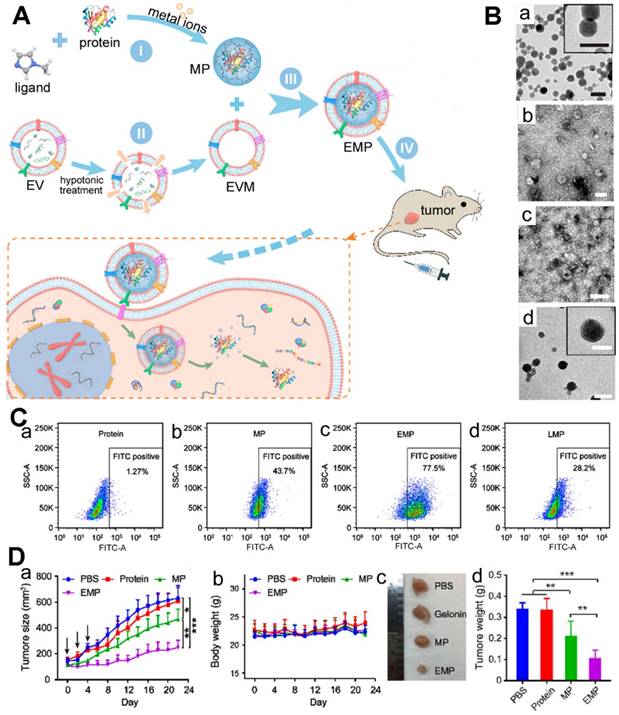
(A) Schematic illustration of the EMP nanoparticles and the application of the intracellular delivery system. (B) TEM images of MP (a), EVs (b), EVM (c), and EMP (d) nanoparticles, scale bars: 100 nm. (C) The flow cytometry analysis study of the bare protein (a), MP (b), EMP (c) and LMP (d) nanoparticles incubated MDA-MB-231 cells. (D) The tumor growth curves (a), body weight variation (b) after injected intravenously with PBS, gelonin, MP and EMP nanoparticles (n = 5). *P < 0.05, **P < 0.01. The MDA-MB-231 xenograft tumors images (c) and the weights (d) after different treatments [88]. Copyright 2018 American Chemical Society.
The MOFs-based biomineralization delivery system was also investigated for cancer-associated theranostic proteins. Caspase 3 could induce the death of cells [84-86], and prior work co-encapsulated caspase 3/HSA in ZIF-8 NPs with a further PVP coating [75, 87]. When the HeLa cells were treated with caspase 3/HSA@MOF hybrid, the caspase 3 could be delivered to the cancer cells, and the cell plasma membrane was damaged. Further cell viability studies suggested that the caspase 3/HSA@MOF hybrid had obvious cytotoxicity that varied with concentration; no cytotoxicity was found for the HSA@MOF hybrid.
In another example, Cheng et al. also encapsulated protein using the biomineralization of ZIF-8 (Figure 3) [88]. With the modified procedure, the encapsulation efficiency of the MOF-protein (MP) was 94%, and the protein loading capacity was as high as 41%. This was 3 to 50 times that of surface conjunction or adsorption loading. The biomineralized MOFs retained their nanostructure in PBS (pH = 7.4) but degraded in acidic buffer (pH = 5.0) after 1 h with the cargo releasing. Previous studies have demonstrated that extracellular vesicle (EV) could enhance blood circulation time, reduce phagocyte uptake, and preferentially gather in the homotypic tumor area [89-93]. These authors coated EVs on the surface of MP nanoparticles to increase the physiological solution stability and then used them for cancer therapy. In MDA-MB-231 cells, the pure gelonin showed low protein transduction efficiency (∼1.27%), but the MP nanoparticles obviously promoted the transduction efficiency (∼43.7%). Furthermore, after coated with EV, the prepared EV-MOF-protein (EMP) enabled the transduction efficiency increased to ∼77.5%, far higher than the liposome-enveloped MP nanoparticles. An in vivo study with MDA-MB-231 tumor-bearing mice demonstrated that the multifunctional MOFs could accumulate in the tumor area within 24 h post-injection (iv). The mice treated with EMP nanoparticles could effectively inhibit the tumor growth than the other groups and the collected tumors were less than the other groups, implying the good protein delivery effect of the prepared biomineralization system.
Protein-MOFs possess the following advantages: i) The biomineralized MOFs have an extremely high protein loading capacity relative to the surface modification; ii) The MOFs protect therapeutic protein from enzyme degradation in the circulation and tumor microenvironment, and maintained the bioactivity in the cancer cells; iii) The MOFs can degrade in the weakly acidic solution, leading to the encapsulated proteins being released from the MOFs structure in the acidic endo-lysosomes. The biomimetic MOF system needed minimum dosage of the therapeutic proteins and showed good cancer therapy. This was a significant platform for other therapeutic proteins leading to clinic applications of the protein-based nanomaterial.
(2) Antigen and Antibody
Cancer immunotherapy-based antibodies and/or antigens are important in cancer treatment [94-100]. Nano-carrier gave vaccines more effective immune response than pure protein antibody/antigen [101, 102]. The antigen-presenting dendritic cells (DCs) can present the danger signal to T-lymphocyte and generating the CD4+ and CD8+ T cells, resulting of the cancer immunotherapy and induce the “immune memory” effect [103, 104]. The nano-carrier can “programming” the activation state of DCs and induce the cellular and humoral immunity [105]. In studies of antigen-induced immunotherapy, ovalbumin (OVA) is a common model antigen [106, 107]. In 2012, Irvine's group used lipid-coated poly(lactic-co-glycolic acid) (PLGA) NPs as efficient carrier of OVA, and combined this with the adjuvant of the nano-vaccine to elicit three orders of magnitude higher serum anti-OVA IgG titers versus pure soluble OVA [108].
The biomineralized MOF system is also a good potential carrier of the protein antigen as first described by Qu et al [109]. The OVA was encapsulated into the ZIF-8 NPs with a loading capacity of 7%, and then the cytosine-phosphate-guanine oligodeoxynucleotides (CpG ODNs) was adsorbed on the surface of OVA@ZIF-8. Afterwards, in the group of RAW264.7 cells treated with OVA@ZIF-8-CPG, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) had much higher expression versus the other groups, implying the enhanced immunostimulatory activity of the nanocomposites. Later, an in vivo immune response of OVA@ZIF-8-CPG was studied after the nanocomposites subcutaneously injected into Kunming mice. The anti-OVA IgG titers were much higher than the OVA/CPG mixture. In addition, the CD4+ T and CD8+ T cells after OVA@ZIF-8-CPG treatment were also higher than the OVA/CPG mixture group. These results implied that the antigen-biomineralized MOFs possessed superior loading capacity, efficient delivery, and pH-responsive release for antigen-presenting cells. This led to a strong humoral immune response. A good cancer immunotherapeutic effect was achieved by antigen-biomineralized MOFs.
Moreover, tumors may evade immune destruction by endogenous “immune checkpoints” and terminate the antigen-activated immune responses, the presence of immune inhibitory receptors in T cells [99]. So, the immune-checkpoint-pathway inhibitors such as anti-PD-L1 antibody, anti-PD1 antibody or anti-CTLA-4 antibody can recover the immune responses and develop cancer immunotherapeutic approaches [110-113]. The delivery of the antibody with an efficient strategy and controllable release of antibodies can spare the essential dose of the antibody and decrease the cost of treatment [114]. The antibody biomineralized MOFs could protect the protein against various severe environment, we assume that the antibody biomineralized MOFs can act as prominent delivery strategy of the immune-checkpoint-pathway inhibitors and feature powerful clinical potency in cancer immunotherapy.
(3) Enzyme
For enzyme-induced cancer treatment, glucose oxidase (GOx) could catalyze glucose resulting in the production of gluconic acid and hydrogen peroxide (H2O2) [115-117]. The rapid growth of the tumor was dependent on the glucose supply in the cancer cells, and the starvation therapy could eliminate tumors by the reduction of glucose via GOx [118-121]. In addition, as the hypoxia enhanced, the therapeutic effect of hypoxia-activated prodrugs could increase, and the synergy effect was achieved [122, 123]. Also, the generated H2O2 could produce the hydroxyl radical through the Fenton reaction [124, 125], or oxidize L-Arginine (L-Arg) with a production of NO, resulting of the starvation synergistic cancer therapy [126]. In recent years, the biomineralization of enzyme has been extensively investigated to enhance the enzymatic activity and extend the enzyme's application environment [42, 74, 127].
(A) Schematic illustration of the TGZ@eM and the cancer starvation therapy; (B) the cell viability after treated with different nanoparticles; (C) the HIF-1α staining of MCTS after different treatments; (D) the fluorescence images of MCTS after treated with different nanoparticles ((1) PBS, (2) ZIF-8@eM, (3) GZ@eM, (4) TZ@eM, (5) TGZ@eM) [128]. Copyright 2018 American Chemical Society.
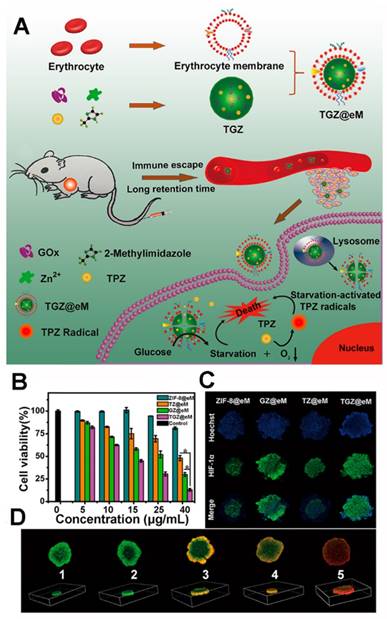
In 2018, Qu's group reported an enzyme@MOF hybrid for the starvation-activated therapy [128]. Through a self-assembly procedure (Figure 4), the GOx and prodrug tirapazamine (TPZ) were encapsulated in the ZIF-8 MOF, and the erythrocyte membrane was further coated on the surface of the MOF hybrid to enhance the tumor accumulation and blood circulation [129-136]. The loading amounts of GOx and TGZ were ~10 wt % and 13.2 wt% respectively, which were high enough for the further study. The GOx and TGZ were released into cytoplasm due to the acidity of the lysosome/endosome environment. The GOx could consume the endogenous glucose and O2 to starve the cancer cells. Concurrently, a hypoxic microenvironment was enhanced and thus the TPZ was transformed into highly cytotoxic radical leading to synergistic therapy.
The TGZ@eM showed high cytotoxicity against CT26 cells than the other groups implying the combined treatment effect. The hypoxia-inducible factor-1α (HIF-1α) staining assays of CT26 multicellular tumor spheroids (MCTS) after incubation with different nanostructures implied the O2 reduction effect of GOx. A LIVE/DEAD kit was used after the TGZ@eM treatment to study cell cytotoxicity, and this was confirmed in the MCTS. The GOx in the TGZ@eM nanoreactor deprived the glucose and O2 in the cancer cells resulting in starvation therapy and the hypoxia-induced chemotherapy. When studied in vivo of CT26 tumor-bearing mice, the TGZ@eM group showed satisfactory therapeutic outcomes, implying the synergistic effect of the GOx-based starvation therapy and the activated TPZ therapy.
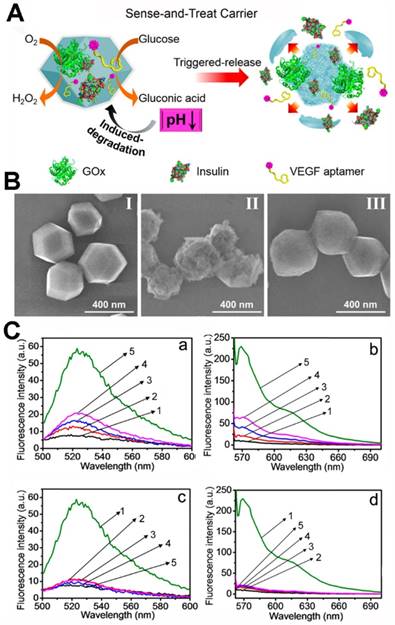
Willner's group co-immobilized insulin/GOx or anti-vascular endothelial growth factor aptamer (VEGF aptamer)/GOx in ZIF-8 NPs (Figure 5) [137]. When the biomineralized MOFs were immersed in the glucose solution, the glucose was transformed to gluconic acid and the acidity of the solution increased. Afterwards, the ZIF-8 nano-mixture was decomposed, and the co-encapsulated insulin or VEGF aptamer was released. And, in the PBS (pH = 7.4), the ZIF-8 nano-mixture stained nanostructure without any change. In a further study, the release of insulin or VEGF was accelerated with the increasing of glucose concentrations. Glucose selectivity studies showed that only glucose could induce the release of insulin or VEGF. The glucose in angio-epithelial cells is higher than the normal cells [138-139], and the VEGF aptamer could inhibit the proliferation and migration of the epithelial cells [140-142]. Thus, the VEGF aptamer/GOx ZIF-8 NPs could respond in the epithelial cells, and the glucose was reduced along with release of the VEGF aptamer. The VEGF aptamer/GOx ZIF-8 NPs showed potential applications in cancer therapy. In the tumor tissues, the reduction of glucose by GOx might damage the cancer cells and epithelial cells via starvation; the released VEGF aptamer could inhibit angiogenesis.
Willner's group also demonstrated that two- or three enzyme cascades possessed better catalytic activity than the enzyme mixture in solution because the MOF nanoreactors provided the enzymes with intercommunication on the nanoscale [143]. The GOx and horseradish peroxidase (HRP) were encapsulated into ZIF-8 NMOFs, and the enzyme cascade was activated with the addition of glucose. The biocatalytic activity of the obtained two-enzyme cascade was 7.5-fold enhanced than the GOx/HRP homogeneous mixture. The β-galactosidase (β-Gal)/GOx/HRP three-enzyme cascade was also evaluated, and the biocatalytic activity 5.3-fold enhanced than the β-Gal/GOx/HRP homogeneous mixture.
(A) The preparation procedure of the multifunctional GOx-loaded ZIF-8 MOFs and the glucose-responsive degradation procedure. (B) SEM images of insulin/GOx@MOFs (II), MOFs after reacted with glucose (50 mM) for 1 h (III) and MOFs incubated in PBS (pH 7.4) for 2 days. (C) Glucose-induced release from the insulin/GOx@MOFs (a) and VEGF aptamer/GOx@MOFs (b) in the presence of glucose ((1) 0 mM, (2) 1 mM, (3) 5 mM, (4) 10 mM, (5) 50 mM); selective glucose-induced release from the insulin/GOx@MOFs (c) and VEGF aptamer/GOx@MOFs (d) in the presence of glucose (1), galactose (2), β-lactose (3), sucrose (4), pure buffer solution (5) [137]. Copyright 2018 American Chemical Society.
MOFs that possessed stable nanostructure and enhanced enzyme catalytic activity in the endo-lysosome or cytoplasm might be another approach to the cancer treatment [144-148]. We assume that enzyme biomineralized MOFs can be applied to cancer therapy in two ways: (i) the enzyme-MOFs decompose in the tumor microenvironment and thus the enzyme show catalytic effects combine with therapy of the other released drug (the MOFs decomposed due to the catalytic effect and the co-encapsulated drug was released); (ii) the enzyme-MOFs show enhanced catalytic effect in tumor tissues and influence the endo-biochemical system leading to the therapeutic effect.
3. MOFs-based biomineralization of DNA/RNA
The cancer gene therapy based on the RNA or DNA has attracted significant attention in the past two decades [149-152].The RNA interference (RNAi) has been applied to the sequence specific silencing of target messenger RNA (mRNA), and thus decrease the suppression of gene and protein, resulting of the gene therapy [153-154]. And, the synthetic short interfering RNAs (siRNAs) and plasmid DNA (pDNA) are the most widely studied therapeutic drugs for the RNAi [155]. However, these gene drugs are large biomacromolecules, cannot efficiently delivered to the tumor tissues and cross the cell membrane to cytoplasm, and in the serum can be degraded by the nuclease. The current major strategies to delivery RNA or DNA are delivery nano-vehicles, including various cationic materials such as liposomal, polymeric formulations and inorganic nanoparticles [156]. There are some problems for these common delivery systems, such as suboptimal transfection effect and the potential hemolytic effect [157].
MOFs also can be applied in gene delivery system [158-159]. Mirkin and the other researchers recently prepared DNA-MOFs using click chemistry to conjugate the dibenzocyclooctyne-functioned DNA (DNA-N3), azide-functioned MOFs (MOFs-N3) [160,161], or coordination chemistry between the phosphate of DNA and the external metal nodes (Zr, Hf, Fe) of MOFs [162-164]. Lin and other researchers loaded the RNA on the surface of MOFs via metal ion connections or physical absorption [33, 164].
However, the complicated modification of DNA/RNA and the lack of release stimuli-environmental limit its application to gene therapy. Coating the DNA/RNA with non-toxic inorganic shells can leave out the DNA/RNA modification and extend the delivery system while keeping the length of the DNA/RNA unconstrained. In addition, the biomineralization can prolong the shelf life time and offer possibilities for clinical applications of genomic drugs [11].
Recently, calcium phosphate (CaP) has been used to coat living agents due to its good biocompatibility, stability, non-immunogenicity, and pH-responsive decomposition in the tumor tissues [165]. And, the CaP can be used as the biomineral shells of the DNA/RNA via the interfacial interactions between calcium and phosphate ions, and the DNA/RNA can release due to the pH in the tumor cells [166, 167]. But the traditional biomineralization coating was critical to their surface, and direct biomineralization of the genetic macromolecules was difficult to realize. However, Liang et al. encapsulated DNA using ZIF-8 with a high encapsulation efficiency of 75% implying that the MOFs could be potentially applied as an effective bioactive DNA carrier [11].
(A) Biomimetic Cas9/sgRNA@MOF hybrid for genome editing: (a) schematic illustration of the Cas9/sgRNA encapsulated within ZIF-8; the Flow cytometry analysis (B) and qPCR quantitation (c) of different treatments 2 and 4 days [168]. Copyright 2018 American Chemical Society.
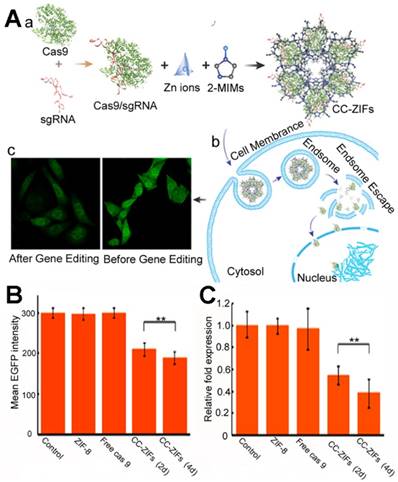
Khashab et al. first reported RNA-biomineralized MOFs [168]. The site-specific gene editing CRISPR/Cas9 platform was incorporated with a Cas9 protein and single guide RNA (sgRNA) for cancer treatment [169-171]. The co-delivery of the Cas9 protein or mRNA and sgRNA was necessary to translate this into the internal environment. To co-encapsulate the Cas9 protein and sgRNA, these authors encapsulated the CRISPR/Cas9 platform with ZIF-8 (Figure 6A) and the loading capacity was 1.2 wt% with an encapsulation efficiency of 17%. The pH-responsive nature of the ZIF-8 enabled the endosomal escape of the CRISPR/Cas9@ZIF-8 and the release of the gene editing platform. When the enhanced green fluorescent protein (eGFP) Chinese hamster ovary cells were treated with the eFGP editing CRISPR/Cas9@ZIF-8 nano-composites, the eGFP was reduced after 2 or 4 days, which was far more efficient than the free CRISPR/Cas9. The CRISPR/Cas9@ZIF-8 could also be potentially applied to cancer immunotherapy; this protein and RNA co-biomineralization strategy showed guidance for further RNA or protein-RNA delivery via MOFs.
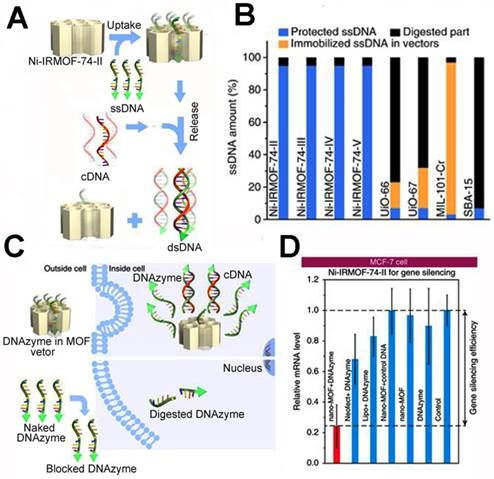
Aside from the in situ biomineralization method, Zhou et al. presented a precise DNA loading method inner the isoreticular MOFs (NiIRMOF-74) [172]. By changing the length of the organic linker, the authors prepared NiIRMOF-74-II to -V with 2.2 to 4.2 nm pore sizes. The pore of the NiIRMOF-74 provided a host-guest interaction to the NiIRMOF-74 and single-stranded DNA (ssDNA). This enabled precise loading of the ssDNA inside the pores. Upon addition of the complementary DNA (cDNA), the ssDNA was released due to the interaction between the ssDNA and cDNA (Figure 7). The loading capacity was up to 6.9 wt%, which is far higher than other MOFs. The ssDNA loaded in NiIRMOF-74 could maintain 95% survival after incubation in FBS for 24 h, while the other porous nanostructures had low ssDNA survival, implying the good protection of NiIRMOF-74 vectors. When the MCF-7 cells were treated with 33-nucleotide ssDNA (DNAzyme)-loaded Ni-IRMOF-74-II, it inhibited 76% EGR-1 gene expression. The precise DNA loading rate and excellent transfection efficiency showed good potential in cancer gene therapy.
4. Other biomineralization
Other than the biomacromolecule, various living bacteria and virus also have been applied in the cancer treatment. Even though the living bacteria and virus are promising in cancer treatment, sometimes the biomedical applications are limited by the host immune antiviral effect and inefficient tumor accumulation [173]. Artificial biomineralization of the living bacteria or virus agent can solve the problem of living agent-based cancer treatment [174]. By coating the living agent surface with a mineral layer or encapsulating several living agents into one mineral nanostructure, the living agent can accumulate in the tumor.
Biomineralization using CaP can be realized through the interactions between calcium ions and amino acids on the surface of the bacteria or virus, followed by the addition of phosphate ions [174, 175]. However, the certain amino acids on the surface of the living agents limited the universality for the strategy, and the morphology of the mineral hybrids need further study to meet the clinical research standard. As the core-shell nanostructure with MOF shell was studied, and combine with the study of the MOFs-based biomineralization, the various MOFs can also be applied in the biomineralization of the living agents.
The precise inclusion of single-stranded DNA using isoreticular MOFs: (A) the ssDNA transfection procedure of ssDNA@Ni-IRMOF-74-II; (B) the protection studies of ssDNA using different porous nanostructures in FBS; (C) the intracellular delivery of ssDNA using ssDNA@Ni-IRMOF-74-II and pure ssDNA; (D) gene silencing efficiency of MCF-7 cells for the DNAzyme delivery with Ni-IRMOF-74-II, Lipo, and Neofec [172]. Copyright 2018 Nature.
(1) Bacteria
Nowadays, the bacteria especially the salmonella has been applied as antitumor agents due to its tumor preferentially amplifying and even 1000 times than the normal tissues [176, 177]. The salmonella can express the prodrug-converting enzymes, such as herpes simplex thymidine kinase. In addition, the salmonella induces the production of the Tumor Necrosis Factor-α (TNF-α) from immune cells, and thus lead to the immune killing of cancer cell. With the development of the bacterial adjuvants, the applications of bacteria in the cancer treatment have gained much research interest [178-181].
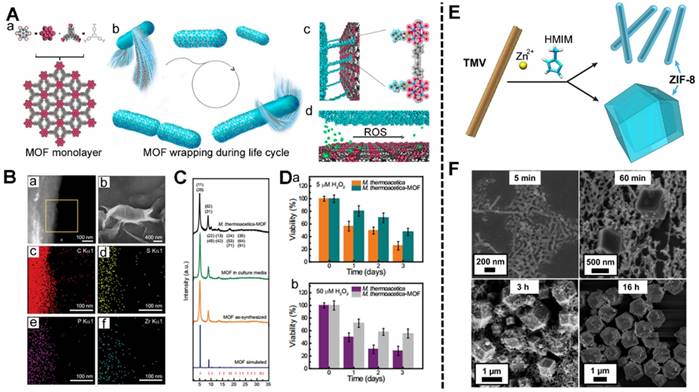
The Yang's group attempted the biomineralization of anaerobic bacteria using MOF based on the interaction between the Zr4+ and the phosphate units on surface of the bacteria [182]. As shown in Figure 8, the Zr6O4(OH)4(BTB)2(OH)6(H2O)6 clusters (BTB = 1,3,5-benzenetribenzoate) was coated on the surface of Moorella thermoacetica (M. thermoacetica) in all its life cycle. And, when treated with the ROS, the viability of M. thermoacetica-MOF was obvious higher than the pure the M. thermoacetica, implying the good protection effect of the coated MOF shell. The efficient coating of the bacteria using MOFs and the protection effect could enable the application of the bacteria@MOFs in cancer treatment. In addition, with the rational design of the organic ligands, the bacteria@MOFs could be prepared to release in the tumor microenvironment or under the external excitation, and thus the bacteria showed therapeutic effect.
(2) Virus
In addition, oncolytic viruses (OVs) including poxvirus, paramyxovirus, reovirus, and picornavirus are the most widely studied among virus immunotherapy [183, 184]. OVs are genetically modified to selectively infect the cancer cells and replicate in the cells regardless of the normal cells. The preclinical OVs have effective antitumor effect [185]. Viral gene delivery systems include adenoviruses (Ads) and lentiviruses. The Gassensmith's group used tobacco mosaic virus (TMV) as a template-fabricated TMV@MOF using ZIF-8 (Figure 8E) [44, 186-187]. The prepared TMV@MOF had a tuned nanostructure via changes in the synthetic conditions. Meanwhile, the surface of the TMV@MOF could also be conjugated with organic molecules or biomolecule via chemical reactions. Later, this group demonstrated that a high zinc concentration near the TMV could catalyze the growth of ZIF-8 on the surface of the TMV. This would benefit the other virus-MOF core-shell systems.
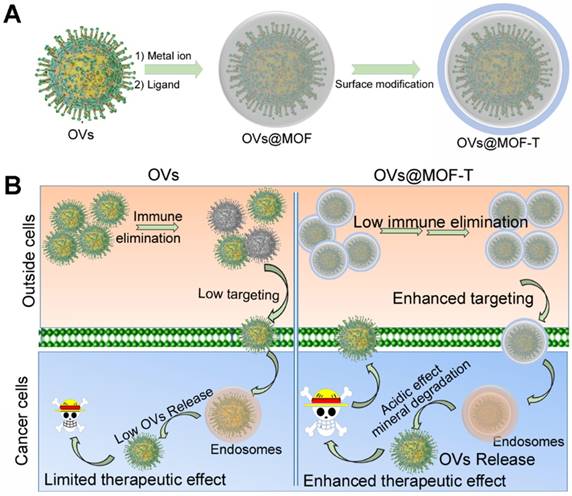
It was supposed that the OVs can be encapsulated into MOFs during direct self-assembly (Figure 9) [68]. And, the surface modification enhances the targeting effect and biocompatibility of the OVs@MOF. Unlike protein or DNA/RNA, the OVs are more difficult to store and transport. MOFs-based biomineralization gives the OVs@MOF a longer lifetime after assembly versus free OVs. In addition, the targeting modified OVs@MOF hybrid (OVs@MOF-T) is more suitable for cancer treatment. The OVs@MOF-T can specifically accumulate in the tumor tissues more efficiently regardless of the immunogenic effect of OVs. Subsequently, OVs are released from the endosomes due to the pH-response MOF disassembly, achieving significant therapeutic efficacy in cancer treatment.
The MOFs-based biomineralization of anaerobic bacteria: (A) the formation of the MOF (a), the M. thermoacetica-MOF wrapping cycle (b), the interface of the MOF and bacteria (c), the ROS response of the interface (d); (B) High-angle annular dark-field STEM image (a) and SEM image (b) of the prepared M. thermoacetica-MOF, EDS mapping of the M. thermoacetica-MOF ((c) C element, (d) S element, (e) P element, (f) Zr element); (C) PXRD pattern and Bragg position of M. thermoacetica-MOF, MOF soaked in culture media, MOF as-synthesized and MOF simulated; (D) The viability of M. thermoacetica and M. thermoacetica-MOF when treated with 5 μM (a), and 50 μM (b) H2O2 [182]. Copyright 2018 National Academy of Sciences. The preparation of TMV@ZIF-8 nanocomposites: (E) the schematic illustration of the TMV@ZIF-8 rod-shaped nanocomposites; (F) the SEM of the TMV@ZIF-8 composites varying the reaction time. [187]. Copyright 2018 American Chemical Society.
The potential encapsulation procedure (A) and the intracellular delivery (B) of OVs using MOF.
5. Conclusions and outlook
MOFs-based biomineralization is useful in biological catalysis or nanomedicine. The biomineralization of protein, DNA/RNA, or viruses can be done with MOF encapsulation. Biotherapy for cancer with this approach has shown progress—the MOFs can be loaded with cancer-associated biomacromolecules. The biomacromolecules maintain their biological activity regardless of the extreme tumor microenvironment and delivery via MOF protection.
For application of the biomineralization towards cancer therapy, the MOF should possess the following properties: (i) the MOF synthetic conditions should be moderately facile, and the MOF should interact with the biomacromolecules and protect them from the surroundings; (ii) the size, biological stability, and biosecurity of the MOF should meet the requirements for cancer treatment nanomedicine; (iii) the MOF should be responsive to the tumor microenvironment and thus the encapsulated biomacromolecules or living agents released. Currently, the preparation method for the MOFs-based biomineralization is mostly limited to the ZIF-8 or ZIF-90, and the organic bridging ligands still have in vivo toxicity. However, better understanding of nano-bio interactions to exploit MOF-architectures for cancer treatment can be adapted or modified to improve their performance and dose efficiency—this reduces patient exposure to the therapeutics.
In summary, the application of a MOF-biomineralized therapeutic protein, antigen/antibody, enzyme, and DNA/RNA leads to improved cancer treatment efficacy. Their properties allow for unique opportunities in modulating key components of the healthy organism system such as the inherent activation of immune cells and functional delivery of biomacromolecules as well as biosensing and monitoring of treatment response. The field of MOF-based cancer treatment combines researchers from multiple subdisciplines of material science, nano-chemistry, and nano-biological interactions, immunology, and medicinal chemistry. This leads to new opportunities to synergistically complement and improve MOF-based cancer nanomedicine.
Acknowledgements
This work was supported by the Major State Basic Research Development Program of China (2017YFA0205201 and 2018YFA0107301), the National Natural Science Foundation of China (81422023, 81603015, 81871404, U1705281, and U1505221), the Fundamental Research Funds for the Central Universities (20720160065 and 20720190088), the Program for New Century Excellent Talents in University, China (NCET-13-0502) and Postdoctoral International Exchange Program [2012]310.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional Nanomaterials for Phototherapies of Cancer. Chem Rev. 2014;114:10869-939
2. Fan W, Yung B, Huang P, Chen X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem Rev. 2017;117:13566-638
3. Chu C, Lin H, Liu H, Wang X, Wang J, Zhang P. et al. Tumor Microenvironment-Triggered Supramolecular System as an In Situ Nanotheranostic Generator for Cancer Phototherapy. Adv Mater. 2017;29:1605928
4. Liu G, Choi KY, Bhirde A, Swierczewska M, Yin J, Lee SW. et al. Sticky Nanoparticles: A Platform for siRNA Delivery by a Bis(zinc(II) dipicolylamine)-Functionalized, Self-Assembled Nanoconjugate. Angew Chem Int Ed. 2011;51:445-9
5. Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016;16:2334-40
6. Lin G, Zhang Y, Zhu C, Chu C, Shi Y, Pang X. et al. Photo-excitable hybrid nanocomposites for image- guided photo/TRAIL synergistic cancer therapy. Biomaterials. 2018;176:60-70
7. Stock N, Biswas S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem Rev. 2012;112:933-69
8. Wang S, McGuirk CM, d'Aquino A, Mason JA, Mirkin CA. Metal-Organic Framework Nanoparticles. Adv Mater. 2018;30:1800202
9. Furukawa H, Cordova KE, O'Keeffe M, Yaghi OM. The Chemistry and Applications of Metal-Organic Frameworks. Science. 2013;341:1230444
10. Zhou H-C, Long JR, Yaghi OM. Introduction to Metal-Organic Frameworks. Chem Rev. 2012;112:673-4
11. Zhou H-CJ, Kitagawa S. Metal-Organic Frameworks (MOFs). Chem Soc Rev. 2014;43:5415-8
12. Li H, Wang K, Sun Y, Lollar CT, Li J, Zhou H-C. Recent advances in gas storage and separation using metal-organic frameworks. Mater Today. 2018;21:108-21
13. Huang Y-B, Liang J, Wang X-S, Cao R. Multifunctional metal-organic framework catalysts: synergistic catalysis and tandem reactions. Chem Soc Rev. 2017;46:126-57
14. Corma A, García H, Llabrés i Xamena FX. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem Rev. 2010;110:4606-55
15. Ahn DY, Lee DY, Shin CY, Bui HT, Shrestha NK, Giebeler L. et al. Novel Solid-State Solar Cell Based on Hole-Conducting MOF-Sensitizer Demonstrating Power Conversion Efficiency of 2.1%. ACS Appl Mater Inter. 2017;9:12930-5
16. Park HJ, So MC, Gosztola D, Wiederrecht GP, Emery JD, Martinson ABF. et al. Layer-by-Layer Assembled Films of Perylene Diimide- and Squaraine-Containing Metal-Organic Framework-like Materials: Solar Energy Capture and Directional Energy Transfer. ACS Appl Mater Inter. 2016;8:24983-8
17. Kreno LE, Leong K, Farha OK, Allendorf M, Van Duyne RP, Hupp JT. Metal-Organic Framework Materials as Chemical Sensors. Chem Rev. 2012;112:1105-25
18. Huang X, He Z, Guo D, Liu Y, Song J, Yung BC. et al. "Three-in-one" Nanohybrids as Synergistic Nanoquenchers to Enhance No-Wash Fluorescence Biosensors for Ratiometric Detection of Cancer Biomarkers. Theranostics. 2018;8:3461-73
19. Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S. et al. Diverse Applications of Nanomedicine. ACS Nano. 2017;11:2313-81
20. Meng X, Gui B, Yuan D, Zeller M, Wang C. Mechanized azobenzene-functionalized zirconium metal- organic framework for on-command cargo release. Sci Adv. 2016;2:e1600480
21. Wang D, Zhou J, Shi R, Wu H, Chen R, Duan B. et al. Biodegradable Core-shell Dual-Metal-Organic-Frameworks Nanotheranostic Agent for Multiple Imaging Guided Combination Cancer Therapy. Theranostics. 2017;7:4605-17
22. Liang K, Ricco R, Doherty CM, Styles MJ, Bell S, Kirby N. et al. Biomimetic mineralization of metal- organic frameworks as protective coatings for biomacromolecules. Nat Commun. 2015;6:7240
23. He C, Liu D, Lin W. Nanomedicine Applications of Hybrid Nanomaterials Built from Metal-Ligand Coordination Bonds: Nanoscale Metal-Organic Frameworks and Nanoscale Coordination Polymers. Chem Rev. 2015;115:11079-108
24. Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T. et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2009;9:172-8
25. Lu K, Aung T, Guo N, Weichselbaum R, Lin W. Nanoscale Metal-Organic Frameworks for Therapeutic, Imaging, and Sensing Applications. Adv Mater. 2018;30:1707634
26. Cai W, Chu C-C, Liu G, Wáng Y-XJ. Metal-Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small. 2015;11:4806-22
27. Zhou J, Tian G, Zeng L, Song X, Bian X-w. Nanoscaled Metal-Organic Frameworks for Biosensing, Imaging, and Cancer Therapy. Adv Healthc Mater. 2018;7:1800022
28. Li B, Wang X, Chen L, Zhou Y, Dang W, Chang J. et al. Ultrathin Cu-TCPP MOF nanosheets: a new theragnostic nanoplatform with magnetic resonance/near-infrared thermal imaging for synergistic phototherapy of cancers. Theranostics. 2018;8:4086-96
29. Liu W-L, Wu C-Y, Chen C-Y, Singco B, Lin C-H, Huang H-Y. Fast Multipoint Immobilized MOF Bioreactor. Chem-Eur J. 2014;20:8923-8
30. Chen Q, Xu M, Zheng W, Xu T, Deng H, Liu J. Se/Ru-Decorated Porous Metal-Organic Framework Nanoparticles for The Delivery of Pooled siRNAs to Reversing Multidrug Resistance in Taxol-Resistant Breast Cancer Cells. ACS Appl Mater Inter. 2017;9:6712-24
31. Jung S, Park S. Dual-Surface Functionalization of Metal-Organic Frameworks for Enhancing the Catalytic Activity of Candida antarctica Lipase B in Polar Organic Media. ACS Catal. 2017;7:438-42
32. Doherty CM, Grenci G, Riccò R, Mardel JI, Reboul J, Furukawa S. et al. Combining UV Lithography and an Imprinting Technique for Patterning Metal-Organic Frameworks. Adv Mater. 2013;25:4701-5
33. He C, Lu K, Liu D, Lin W. Nanoscale Metal-Organic Frameworks for the Co-Delivery of Cisplatin and Pooled siRNAs to Enhance Therapeutic Efficacy in Drug-Resistant Ovarian Cancer Cells. J Am Chem Soc. 2014;136:5181-4
34. Deng H, Grunder S, Cordova KE, Valente C, Furukawa H, Hmadeh M. et al. Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science. 2012;336:1018-23
35. Chen Y, Han S, Li X, Zhang Z, Ma S. Why Does Enzyme Not Leach from Metal-Organic Frameworks (MOFs)? Unveiling the Interactions between an Enzyme Molecule and a MOF. Inorg Chem. 2014;53:10006-8
36. Chen Y, Lykourinou V, Vetromile C, Hoang T, Ming L-J, Larsen RW. et al. How Can Proteins Enter the Interior of a MOF? Investigation of Cytochrome c Translocation into a MOF Consisting of Mesoporous Cages with Microporous Windows. J Am Chem Soc. 2012;134:13188-91
37. Lykourinou V, Chen Y, Wang X-S, Meng L, Hoang T, Ming L-J. et al. Immobilization of MP-11 into a Mesoporous Metal-Organic Framework, MP-11@mesoMOF: A New Platform for Enzymatic Catalysis. J Am Chem Soc. 2011;133:10382-5
38. He H, Han H, Shi H, Tian Y, Sun F, Song Y. et al. Construction of Thermophilic Lipase-Embedded Metal-Organic Frameworks via Biomimetic Mineralization: A Biocatalyst for Ester Hydrolysis and Kinetic Resolution. ACS Appl Mater Inter. 2016;8:24517-24
39. Lu G, Li S, Guo Z, Farha OK, Hauser BG, Qi X. et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat Chem. 2012;4:310-6
40. Lyu F, Zhang Y, Zare RN, Ge J, Liu Z. One-Pot Synthesis of Protein-Embedded Metal-Organic Frameworks with Enhanced Biological Activities. Nano Lett. 2014;14:5761-5
41. Doonan C, Riccò R, Liang K, Bradshaw D, Falcaro P. Metal-Organic Frameworks at the Biointerface: Synthetic Strategies and Applications. Acc Chem Res. 2017;50:1423-32
42. Lian X, Fang Y, Joseph E, Wang Q, Li J, Banerjee S. et al. Enzyme-MOF (metal-organic framework) composites. Chem Soc Rev. 2017;46:3386-401
43. Nath I, Chakraborty J, Verpoort F. Metal organic frameworks mimicking natural enzymes: a structural and functional analogy. Chem Soc Rev. 2016;45:4127-70
44. Li S, Dharmarwardana M, Welch RP, Ren Y, Thompson CM, Smaldone RA. et al. Template-Directed Synthesis of Porous and Protective Core-Shell Bionanoparticles. Angew Chem Int Ed. 2016;55:10691-6
45. Walsh G. Biopharmaceutical benchmarks 2018. Nat Biotechnol. 2018;36:1136-45
46. Gaber M, Medhat W, Hany M, Saher N, Fang JY, Elzoghby A. Protein-lipid nanohybrids as emerging platforms for drug and gene delivery: Challenges and outcomes. J Control Release. 2017;254:75-91
47. Kintzing JR, Interrante MVF, Cochran JR. Emerging Strategies for Developing Next-Generation Protein Therapeutics for Cancer Treatment. Trends Pharmacol Sci. 2016;37:993-1008
48. Sanchez-Garcia L, Martín L, Mangues R, Ferrer-Miralles N, Vázquez E, Villaverde A. Recombinant pharmaceuticals from microbial cells: a 2015 update. Microb Cell Fact. 2016;15:1-7
49. Zhou L, Xu E, Sun Y, Liu XM. Targeted biopharmaceuticals for cancer treatment. Cancer Lett. 2014;352:145-51
50. Hsiao YC, Chu LJ, Chen JT, Yeh TS, Yu JS. Proteomic profiling of the cancer cell secretome: informing clinical research. Expert Rev Proteomic. 2017;14:737-56
51. Aes VD, Van GN, Phh B, Rrm J, Friedrich H, Gil-Carton D. et al. Molecular nucleation mechanisms and control strategies for crystal polymorph selection. Nature. 2018;556:89-94
52. Secretin-repligen. SecreFlo. Drugs R D. 2002;3:217-9
53. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581-611
54. Tovar JM, Bazaldua OV, Leticia V, Erin R. Human papillomavirus, cervical cancer, and the vaccines. Postgrad Med. 2008;120:109-11
55. Zimmerman ES, Heibeck TH, Gill A, Li X, Murray CJ, Madlansacay MR. et al. Production of site-specific antibody-drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjugate Chem. 2014;25:351-61
56. Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973-81
57. David B, Alan K, Ronald P, David F, Nils L, Renzo C. The development of immunomodulatory monoclonal antibodies as a new therapeutic modality for cancer: the Bristol-Myers Squibb experience. Pharmacol Therapeut. 2015;148:132-53
58. Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67:95-106
59. Martin S, Makoto T, Wirth LJ, Bruce R, Brose MS, Rossella E. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Eng J Med. 2015;372:621
60. Inman BA, Longo TA, Ramalingam S, Harrison MR. Atezolizumab: a PD-L1 blocking antibody for bladder cancer. Clin Cancer Res. 2017;23:1886-90
61. Jago C. Checkpoint inhibitors: a cutting edge in oncology. Drug Today. 2017;53:399-404
62. Lamb YN. Inotuzumab Ozogamicin: First Global Approval. Drugs. 2017;77:1603-1610
63. Roberts CJ. Therapeutic protein aggregation: mechanisms, design, and control. Trends Biotechnol. 2014;32:372-80
64. Wim J, Theodore W R, David B V, C Russell M, Christian Sn, Gerhard W. et al. Protein instability and immunogenicity: roadblocks to clinical application of injectable protein delivery systems for sustained release. J Pharm Sci. 2012;101:946-54
65. Roberts CJ. Non-native protein aggregation kinetics. Biotechnol Bioeng. 2010;98:927-38
66. Du Y, Gao J, Zhou L, Ma Li, He Y, Huang Z, Jiang Y. Enzyme nanocapsules armored by metal-organic frameworks: A novel approach for preparing nanobiocatalyst. Chem Eng J. 2017;327:1192-7
67. Jeong G-Y, Ricco R, Liang K, Ludwig J, Kim J-O, Falcaro P. et al. Bioactive MIL-88A Framework Hollow Spheres via Interfacial Reaction In-Droplet Microfluidics for Enzyme and Nanoparticle Encapsulation. Chem Mater. 2015;27:7903-9
68. Feng Y, Wang H, Zhang S, Zhao Y, Gao J, Zheng Y. et al. Antibodies@MOFs: An In Vitro Protective Coating for Preparation and Storage of Biopharmaceuticals. Adv Mater. 2018. 1805 148
69. Cai W, Wang J, Chu C, Chen W, Wu C, Liu G. Metal-Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Adv Sci. 2018. 1801 526
70. Yang Y, Hu Q, Zhang Q, Jiang K, Lin W, Yang Y. et al. A Large Capacity Cationic Metal-Organic Framework Nanocarrier for Physiological pH Responsive Drug Delivery. Mol Pharmaceut. 2016;13:2782-6
71. Lin W, Hu Q, Jiang K, Yang Y, Yang Y, Cui Y. et al. A porphyrin-based metal-organic framework as a pH-responsive drug carrier. J Solid State Chem. 2016;237:307-12
72. Zhu Y-D, Chen S-P, Zhao H, Yang Y, Chen X-Q, Sun J. et al. PPy@MIL-100 Nanoparticles as a pH- and Near-IR-Irradiation-Responsive Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. ACS Appl Mater Inter. 2016;8:34209-17
73. Zhao J, Yang Y, Han X, Liang C, Liu J, Song X. et al. Redox-Sensitive Nanoscale Coordination Polymers for Drug Delivery and Cancer Theranostics. ACS Appl Mater Inter. 2017;9:23555-63
74. Shieh F-K, Wang S-C, Yen C-I, Wu C-C, Dutta S, Chou L-Y. et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal-Organic Framework Microcrystals. J Am Chem Soc. 2015;137:4276-9
75. Chen T-T, Yi J-T, Zhao Y-Y, Chu X. Biomineralized Metal-Organic Framework Nanoparticles Enable Intracellular Delivery and Endo-Lysosomal Release of Native Active Proteins. J Am Chem Soc. 2018;140:9912-20
76. Vermonden T, Censi R, Hennink WE. Hydrogels for Protein Delivery. Chem Rev. 2012;112:2853-88
77. Kojima C, Kameyama R, Yamada M, Ichikawa M, Waku T, Handa A. et al. Ovalbumin Delivery by Guanidine-Terminated Dendrimers Bearing an Amyloid-Promoting Peptide via Nanoparticle Formulation. Bioconjugate Chem. 2015;26:1804-10
78. Bhatnagar S, Chawla SR, Kulkarni OP, Venuganti VVK. Zein Microneedles for Transcutaneous Vaccine Delivery: Fabrication, Characterization, and in Vivo Evaluation Using Ovalbumin as the Model Antigen. ACS Omega. 2017;2:1321-32
79. Sun Z, Liang J, Dong X, Wang C, Kong D, Lv F. Injectable Hydrogels Coencapsulating Granulocyte-Macrophage Colony-Stimulating Factor and Ovalbumin Nanoparticles to Enhance Antigen Uptake Efficiency. ACS Appl Mater Inter. 2018;10:20315-25
80. Lemke J, von Karstedt S, Abd El Hay M, Conti A, Arce F, Montinaro A. et al. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ. 2013;21:491-502
81. Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782-98
82. Oh Y, Swierczewska M, Kim TH, Lim SM, Eom HN, Park JH. et al. Delivery of tumor-homing TRAIL sensitizer with long-acting TRAIL as a therapy for TRAIL-resistant tumors. J Control Release. 2015;220:671-81
83. Guimarães PPG, Gaglione S, Sewastianik T, Carrasco RD, Langer R, Mitchell MJ. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano. 2018;12:912-31
84. Ingles J, Simpson A, Kyathanahalli C, Anamthathmakula P, Hassan S, Jeyasuria P. et al. Preconditioning the uterine unfolded protein response maintains non-apoptotic Caspase 3-dependent quiescence during pregnancy. Cell Death Dis. 2018;9:933
85. Chung SW, Cho YS, Choi JU, Kim HR, Won TH, Kim SY. et al. Highly potent monomethyl auristatin E prodrug activated by caspase-3 for the chemoradiotherapy of triple-negative breast cancer. Biomaterials. 2019;192:109-17
86. Vince JE, De Nardo D, Gao W, Vince AJ, Hall C, McArthur K. et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation. Cell Rep. 2018;25:2339-53.e4
87. Shi Y. A structural view of mitochondria-mediated apoptosis. Nat Struct Biol. 2001;8:394-401
88. Cheng G, Li W, Ha L, Han X, Hao S, Wan Y. et al. Self-Assembly of Extracellular Vesicle-like Metal- Organic Framework Nanoparticles for Protection and Intracellular Delivery of Biofunctional Proteins. J Am Chem Soc. 2018;140:7282-91
89. Wan S, Zhang L, Wang S, Liu Y, Wu C, Cui C. et al. Molecular Recognition-Based DNA Nanoassemblies on the Surfaces of Nanosized Exosomes. J Am Chem Soc. 2017;139:5289-92
90. Armstrong JPK, Holme MN, Stevens MM. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano. 2017;11:69-83
91. Ziaei P, Berkman CE, Norton MG. Review: Isolation and Detection of Tumor-Derived Extracellular Vesicles. ACS Appl Nano Mater. 2018;1:2004-20
92. Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano. 2018;12:6830-42
93. Jc Bose R, Uday Kumar S, Zeng Y, Afjei R, Robinson E, Lau K. et al. Tumor Cell-Derived Extracellular Vesicle-Coated Nanocarriers: An Efficient Theranostic Platform for the Cancer-Specific Delivery of Anti-miR-21 and Imaging Agents. ACS Nano. 2018;12:10817-32
94. Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Syntetic Nanoparticles for Vaccines and Immunotherapy. Chem Rev. 2015;115:11109-46
95. Cheng K, Ding Y, Zhao Y, Ye S, Zhao X, Zhang Y. et al. Sequentially Responsive Therapeutic Peptide Assembling Nanoparticles for Dual-Targeted Cancer Immunotherapy. Nano Lett. 2018;18:3250-8
96. Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y. et al. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano. 2018;12:5121-9
97. Cui X-x, Ding H-m, Gu F, Lv Y-y, Xing X, Zhang R. Inhibition of CTHRC-1 by its specific monoclonal antibody attenuates cervical cancer cell metastasis. Biomed Pharmacother. 2019;110:758-63
98. Lee H, Lee JH, Kim J, Mun JH, Chung J, Koo H. et al. Hyaluronate-Gold Nanorod/DR5 Antibody Complex for Noninvasive Theranosis of Skin Cancer. ACS Appl Mater Inter. 2016;8:32202-10
99. Sun L, Chen L, Li H. Checkpoint-modulating immunotherapies in tumor treatment: Targets, drugs, and mechanisms. Int Immunopharmacol. 2019;67:160-75
100. Kisalu NK, Idris AH, Weidle C, Flores-Garcia Y, Flynn BJ, Sack BK. et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med. 2018;24:408-16
101. Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-Based Immunotherapy for Cancer. ACS Nano. 2015;9:16-30
102. Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem Rev. 2015;115:11109-46
103. Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984-93
104. Steinman RM. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155-59
105. Wang K, Wen S, He L, Li A, Li Y, Dong H. et al. “Minimalist” Nanovaccine Constituted from Near Whole Antigen for Cancer Immunotherapy. ACS Nano. 2018;12:6398-409
106. Meng C, Zhi X, Li C, Li C, Chen Z, Qiu X. et al. Graphene Oxides Decorated with Carnosine as an Adjuvant To Modulate Innate Immune and Improve Adaptive Immunity in Vivo. ACS Nano. 2016;10:2203-13
107. Xiang J, Xu L, Gong H, Zhu W, Wang C, Xu J. et al. Antigen-Loaded Upconversion Nanoparticles for Dendritic Cell Stimulation, Tracking, and Vaccination in Dendritic Cell-Based Immunotherapy. ACS Nano. 2015;9:6401-11
108. Bershteyn A, Hanson MC, Crespo MP, Moon JJ, Li AV, Suh H. et al. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J. Control Release. 2012;157:354-65
109. Zhang Y, Wang F, Ju E, Liu Z, Chen Z, Ren J. et al. Metal-Organic-Framework-Based Vaccine Platforms for Enhanced Systemic Immune and Memory Response. Adv Funct Mater. 2016;26:6454-61
110. Sullivan RJ, Flaherty KT. Anti-PD-1 therapies—a new first-line option in advanced melanoma. Nat Rev Clin Oncol. 2015;12:625-6
111. Liu Y, Zheng P. How Does an Anti-CTLA-4 Antibody Promote Cancer Immunity? Trends Immunol. 2018;39:953-6
112. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Eng J Med. 2012;366:2443-54
113. Duperret EK, Trautz A, Stoltz R, Patel A, Wise MC, Perales-Puchalt A. et al. Synthetic DNA-Encoded Monoclonal Antibody Delivery of Anti-CTLA-4 Antibodies Induces Tumor Shrinkage In Vivo. Cancer Res. 2018;78:6363-70
114. Du Y, Liang X, Li Y, Sun T, Jin Z, Xue H. et al. Nuclear and Fluorescent Labeled PD-1-Liposome-DOX-64Cu/IRDye800CW Allows Improved Breast Tumor Targeted Imaging and Therapy. Mol Pharm. 2017;14:3978-86
115. Kirsch J, Siltanen C, Zhou Q, Revzin A, Simonian A. Biosensor technology: recent advances in threat agent detection and medicine. Chem Soc Rev. 2013;42:8733-68
116. Wong CM, Wong KH, Chen XD. Glucose oxidase: natural occurrence, function, properties and industrial applications. Appl Microbiol Biotechnol. 2008;78:927-38
117. Fu L-H, Qi C, Lin J, Huang P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem Soc Rev. 2018;47:6454-72
118. Zhou J, Li M, Hou Y, Luo Z, Chen Q, Cao H. et al. Engineering of a Nanosized Biocatalyst for Combined Tumor Starvation and Low-Temperature Photothermal Therapy. ACS Nano. 2018;12:2858-72
119. Zhao W, Hu J, Gao W. Glucose Oxidase-Polymer Nanogels for Synergistic Cancer-Starving and Oxidation Therapy. ACS Appl Mater Inter. 2017;9:23528-35
120. Li S-Y, Cheng H, Xie B-R, Qiu W-X, Zeng J-Y, Li C-X. et al. Cancer Cell Membrane Camouflaged Cascade Bioreactor for Cancer Targeted Starvation and Photodynamic Therapy. ACS Nano. 2017;11:7006-18
121. Chang K, Liu Z, Fang X, Chen H, Men X, Yuan Y. et al. Enhanced Phototherapy by Nanoparticle-Enzyme via Generation and Photolysis of Hydrogen Peroxide. Nano Lett. 2017;17:4323-9
122. Zhang R, Feng L, Dong Z, Wang L, Liang C, Chen J. et al. Glucose & oxygen exhausting liposomes for combined cancer starvation and hypoxia-activated therapy. Biomaterials. 2018;162:123-31
123. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393-410
124. Chang K, Liu Z, Fang X, Chen H, Men X, Yuan Y. et al. Enhanced Phototherapy by Nanoparticle- Enzyme via Generation and Photolysis of Hydrogen Peroxide. Nano Lett. 2017;17:4323-9
125. Huo M, Wang L, Chen Y, Shi J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun. 2017;8:357
126. Fan W, Lu N, Huang P, Liu Y, Yang Z, Wang S. et al. Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew Chem Int Ed. 2016;56:1229-33
127. Wang Q, Zhang X, Huang L, Zhang Z, Dong S. GOx@ZIF-8(NiPd) Nanoflower: An Artificial Enzyme System for Tandem Catalysis. Angew Chem Int Ed. 2017;56:16082-5
128. Zhang L, Wang Z, Zhang Y, Cao F, Dong K, Ren J. et al. Erythrocyte Membrane Cloaked Metal-Organic Framework Nanoparticle as Biomimetic Nanoreactor for Starvation-Activated Colon Cancer Therapy. ACS Nano. 2018;12:10201-11
129. Shao J, Abdelghani M, Shen G, Cao S, Williams DS, van Hest JCM. Erythrocyte Membrane Modified Janus Polymeric Motors for Thrombus Therapy. ACS Nano. 2018;12:877-85
130. Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT. et al. Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization. Adv Mater. 2017;29:1606209
131. Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220:600-7
132. Wu Z, Li T, Gao W, Xu T, Jurado-Sánchez B, Li J. et al. Cell-Membrane-Coated Synthetic Nanomotors for Effective Biodetoxification. Adv Funct Mater. 2015;25:3881-7
133. Gao W, Hu C-MJ, Fang RH, Luk BT, Su J, Zhang L. Surface Functionalization of Gold Nanoparticles with Red Blood Cell Membranes. Adv Mater. 2013;25:3549-53
134. Li J, Angsantikul P, Liu W, Esteban-Fernández de Ávila B, Chang X, Sandraz E. et al. Biomimetic Platelet-Camouflaged Nanorobots for Binding and Isolation of Biological Threats. Adv Mater. 2018;30:1704800
135. Zhang P, Chen Y, Zeng Y, Shen C, Li R, Guo Z. et al. Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc Natl Acad Sci USA. 2015;112:E6129
136. Zhang P, Zhang L, Qin Z, Hua S, Guo Z, Chu C. et al. Genetically Engineered Liposome-like Nanovesicles as Active Targeted Transport Platform. Adv Mater. 2018;30:1705350
137. Chen W-H, Luo G-F, Vázquez-González M, Cazelles R, Sohn YS, Nechushtai R. et al. Glucose- Responsive Metal-Organic-Framework Nanoparticles Act as “Smart” Sense-and-Treat Carriers. ACS Nano. 2018;12:7538-45
138. Fernando K, Yang H-W, Jiang Y, Jeon Y-J, Ryu B. Diphlorethohydroxycarmalol Isolated from Ishige okamurae Represses High Glucose-Induced Angiogenesis In Vitro and In Vivo. Mar Drugs. 2018;16:375
139. Gu CJ, Xie F, Zhang B, Yang HL, Cheng J, He YY. et al. High Glucose Promotes Epithelial-Mesenchymal Transition of Uterus Endometrial Cancer Cells by Increasing ER/GLUT4-Mediated VEGF Secretion. Cell Physiol Biochem. 2018;50:706-20
140. Ohuchi SP, Shibuya M, Nakamura Y. The RNA Aptamer Inhibiting Human Vesicular Endothelial Growth Factor Receptor 1 without Affecting Cytokine Binding. Biochemistry. 2013;52:2274-9
141. Lee J, Lee BJ, Lee YM, Park H, Kim JH, Kim WJ. Self-Assembled Nanoconstructs Modified with Amplified Aptamers Inhibited Tumor Growth and Retinal Vascular Hyperpermeability via Vascular Endothelial Growth Factor Capturing. Mol Pharmaceut. 2017;14:1460-8
142. Verrax J, Defresne F, Lair F, Vandermeulen G, Rath G, Dessy C. et al. Delivery of Soluble VEGF Receptor 1 (sFlt1) by Gene Electrotransfer as a New Antiangiogenic Cancer Therapy. Mol Pharmaceut. 2011;8:701-8
143. Chen W-H, Vázquez-González M, Zoabi A, Abu-Reziq R, Willner I. Biocatalytic cascades driven by enzymes encapsulated in metal-organic framework nanoparticles. Nat Catal. 2018;1:689-95
144. Tadepalli S, Yim J, Cao S, Wang Z, Naik R R, Singamaneni S. Metal-Organic Framework Encapsulation for the Preservation and Photothermal Enhancement of Enzyme Activity. Small. 2018;14:1702382
145. Li P, Moon S-Y, Guelta MA, Lin L, Gómez-Gualdrón DA, Snurr RQ. et al. Nanosizing a Metal-Organic Framework Enzyme Carrier for Accelerating Nerve Agent Hydrolysis. ACS Nano. 2016;10:9174-82
146. Liang K, Coghlan CJ, Bell SG, Doonan C, Falcaro P. Enzyme encapsulation in zeolitic imidazolate frameworks: a comparison between controlled co-precipitation and biomimetic mineralisation. Chem Commun. 2016;52:473-6
147. Lian X, Huang Y, Zhu Y, Fang Y, Zhao R, Joseph E. et al. Enzyme-MOF Nanoreactor Activates Nontoxic Paracetamol for Cancer Therapy. Angew Chem Int Ed. 2018;57:5725-30
148. Liang Z, Yang Z, Yuan H, Wang C, Qi J, Liu K. et al. A protein@metal-organic framework nanocomposite for pH-triggered anticancer drug delivery. Dalton Trans. 2018;47:10223-8
149. Jeong EH, Kim H, Jang B, Cho H, Ryu J, Kim B. et al. Technological development of structural DNA/RNA-based RNAi systems and their applications. Adv Drug Deliv Rev. 2016;104:29-43
150. Ku SH, Jo SD, Lee YK, Kim K, Kim SH. Chemical and structural modifications of RNAi therapeutics. Adv Drug Deliv Rev. 2016;104:16-28
151. Wang J, Mi P, Lin G, Wáng YXJ, Liu G, Chen X. Imaging-guided delivery of RNAi for anticancer treatment. Adv Drug Deliv Rev. 2016;104:44-60
152. Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396-401
153. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494-8
154. Aagaard L, Rossi JJ. RNAi therapeutics: Principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75-86
155. Lee SH, Chung BH, Park TG, Nam YS, Mok H. Small-Interfering RNA (siRNA)-Based Functional Micro- and Nanostructures for Efficient and Selective Gene Silencing. Acc Chem Res. 2012;45:1014-25
156. Chu C, Ren E, Zhang Y, Yu J, Lin H, Pang X. et al. Zinc(II)-Dipicolylamine Coordination Nanotheranostics: Toward Synergistic Nanomedicine by Combined Photo/Gene Therapy. Angew Chem Int Ed. 2019;58:269-72
157. Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581-93
158. Yang J, Zhang Q, Chang H, Cheng Y. Surface-Engineered Dendrimers in Gene Delivery. Chem Rev. 2015;115:5274-300
159. Wang H-X, Li M, Lee CM, Chakraborty S, Kim H-W, Bao G. et al. CRISPR/Cas9-Based Genome Editing for Disease Modeling and Therapy: Challenges and Opportunities for Nonviral Delivery. Chem Rev. 2017;117:9874-906
160. Chen W-H, Yu X, Liao W-C, Sohn YS, Cecconello A, Kozell A. et al. ATP-Responsive Aptamer-Based Metal-Organic Framework Nanoparticles (NMOFs) for the Controlled Release of Loads and Drugs. Adv Funct Mater. 2017:27 1702102
161. Morris W, Briley WE, Auyeung E, Cabezas MD, Mirkin CA. Nucleic Acid-Metal Organic Framework (MOF) Nanoparticle Conjugates. J Am Chem Soc. 2014;136:7261-4
162. Wang S, McGuirk CM, Ross MB, Wang S, Chen P, Xing H. et al. General and Direct Method for Preparing Oligonucleotide-Functionalized Metal-Organic Framework Nanoparticles. J Am Chem Soc. 2017;139:9827-30
163. Ning W, Di Z, Yu Y, Zeng P, Di C, Chen D. et al. Imparting Designer Biorecognition Functionality to Metal-Organic Frameworks by a DNA-Mediated Surface Engineering Strategy. Small. 2018;14:1703812
164. Wang Z, Fu Y, Kang Z, Liu X, Chen N, Wang Q. et al. Organelle-Specific Triggered Release of Immunostimulatory Oligonucleotides from Intrinsically Coordinated DNA-Metal-Organic Frameworks with Soluble Exoskeleton. J Am Chem Soc. 2017;139:15784-91
165. Chen J, Gao P, Yuan S, Li R, Ni A, Chu L, Ding L, Sun J, Liu X, Duan Y. Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy. ACS Nano. 2016;10:11548-60
166. Choi KY, Silvestre OF, Huang X, Min KH, Howard GP, Hida N. et al. Versatile RNA Interference Nanoplatform for Systemic Delivery of RNAs. ACS Nano. 2014;8:4559-70
167. Wang G, Cao R-Y, Chen R, Mo L, Han J-F, Wang X. et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc Natl Acad Sci USA. 2013;110:7619
168. Alsaiari SK, Patil S, Alyami M, Alamoudi KO, Aleisa FA, Merzaban JS. et al. Endosomal Escape and Delivery of CRISPR/Cas9 Genome Editing Machinery Enabled by Nanoscale Zeolitic Imidazolate Framework. J Am Chem Soc. 2018;140:143-6
169. Nishimasu H, Shi X, Ishiguro S, Gao L, Hirano S, Okazaki S. et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science. 2018;361:1259
170. Joung J, Engreitz JM, Konermann S, Abudayyeh OO, Verdine VK, Aguet F. et al. Genome-scale activation screen identifies a lncRNA locus regulating a gene neighbourhood. Nature. 2017;548:343
171. Hemphill J, Borchardt EK, Brown K, Asokan A, Deiters A. Optical Control of CRISPR/Cas9 Gene Editing. J Am Chem Soc. 2015;137:5642-5
172. Peng S, Bie B, Sun Y, Liu M, Cong H, Zhou W. et al. Metal-organic frameworks for precise inclusion of single-stranded DNA and transfection in immune cells. Nat Commun. 2018;9:1293
173. Wang X, Xiao Y, Hao H, Zhang Y, Xu X, Tang R. Therapeutic Potential of Biomineralization-Based Engineering. Adv Therap. 2018. 1800 079
174. Wang X, Liu X, Xiao Y, Hao H, Zhang Y, Tang R. Biomineralization State of Viruses and Their Biological Potential. Chem-Eur J. 2018;24:11518-29
175. Wang X, Deng Y-Q, Yang D, Xiao Y, Zhao H, Nian Q-G. et al. Biomimetic inorganic camouflage circumvents antibody-dependent enhancement of infection. Chem Sci. 2017;8:8240-6
176. Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M. et al. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37-41
177. Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003;4:548-56
178. Felfoul O, Mohammadi M, Taherkhani S, de Lanauze D, Zhong Xu Y, Loghin D. et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol. 2016;11:941-7
179. Theys J, Landuyt W, Nuyts S, Van Mellaert L, van Oosterom A, Lambin P. et al. Specific targeting of cytosine deaminase to solid tumors by engineered Clostridium acetobutylicum. Cancer Gene Ther. 2001;8:294-7
180. Kasinskas RW, Forbes NS. Salmonella typhimurium Lacking Ribose Chemoreceptors Localize in Tumor Quiescence and Induce Apoptosis. Cancer Res. 2007;67:3201-19
181. Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002;9:291-6
182. Ji Z, Zhang H, Liu H, Yaghi OM, Yang P. Cytoprotective metal-organic frameworks for anaerobic bacteria. Proc Natl Acad Sci USA. 2018;115:10582-7
183. Muharemagic D, Labib M, Ghobadloo SM, Zamay AS, Bell JC, Berezovski MV. Anti-Fab Aptamers for Shielding Virus from Neutralizing Antibodies. J Am Chem Soc. 2012;134:17168-77
184. Jung B-K, Lee YK, Hong J, Ghandehari H, Yun C-O. Mild Hyperthermia Induced by Gold Nanorod- Mediated Plasmonic Photothermal Therapy Enhances Transduction and Replication of Oncolytic Adenoviral Gene Delivery. ACS Nano. 2016;10:10533-43
185. Phan M, Watson MF, Alain T, Diallo J-S. Oncolytic Viruses on Drugs: Achieving Higher Therapeutic Efficacy. ACS Infect Dis. 2018;4:1448-67
186. Riccò R, Liang W, Li S, Gassensmith JJ, Caruso F, Doonan C. et al. Metal-Organic Frameworks for Cell and Virus Biology: A Perspective. ACS Nano. 2018;12:13-23
187. Li S, Dharmarwardana M, Welch RP, Benjamin CE, Shamir AM, Nielsen SO. et al. Investigation of Controlled Growth of Metal-Organic Frameworks on Anisotropic Virus Particles. ACS Appl Mater Inter. 2018;10:18161-9
Author contact
![]() Corresponding author: gangliu.cmitmedu.cn
Corresponding author: gangliu.cmitmedu.cn
 Global reach, higher impact
Global reach, higher impact