13.3
Impact Factor
Theranostics 2019; 9(11):3293-3307. doi:10.7150/thno.32867 This issue Cite
Research Paper
Ferroptosis Promotes Photodynamic Therapy: Supramolecular Photosensitizer-Inducer Nanodrug for Enhanced Cancer Treatment
1. Department of Oral and Maxillofacial-Head Neck Oncology, Department of Laser and Aesthetic Medicine, Shanghai Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China.
2. National Clinical Research Center for Oral Diseases, Shanghai 200011, China.
3. Shanghai Key Laboratory of Stomatology & Shanghai Research Institute of Stomatology, Shanghai 200011, China.
4. School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai 200240, China.
5. Department of Dermatology, Huashan Hospital, Fudan University, Shanghai 200040, China.
6. School of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, China.
#These authors contributed equally to this work.
Abstract
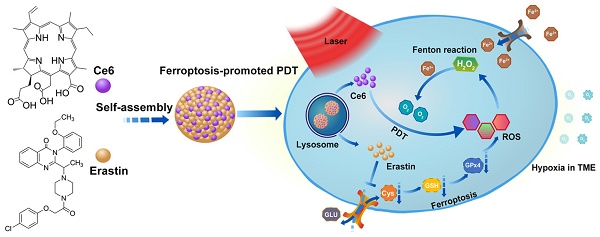
The noninvasive nature of photodynamic therapy (PDT) enables the preservation of organ function in cancer patients. However, PDT is impeded by hypoxia in the tumor microenvironment (TME) caused by high intracellular oxygen (O2) consumption and distorted tumor blood vessels. Therefore, increasing oxygen generation in the TME would be a promising methodology for enhancing PDT. Herein, we proposed a concept of ferroptosis-promoted PDT based on the biochemical characteristics of cellular ferroptosis, which improved the PDT efficacy significantly by producing reactive oxygen species (ROS) and supplying O2 sustainably through the Fenton reaction. In contrast to traditional strategies that increase O2 based on decomposition of limited concentration of hydrogen peroxide (H2O2), our methodology could maintain the concentration of H2O2 and O2 through the Fenton reaction.
Methods: For its association with sensitivity to ferroptosis, solute carrier family 7 member 11 (SLC7A11) expression was characterized by bioinformatics analysis and immunohistochemistry of oral tongue squamous cell carcinoma (OTSCC) specimens. Afterwards, the photosensitizer chlorin e6 (Ce6) and the ferroptosis inducer erastin were self-assembled into a novel supramolecular Ce6-erastin nanodrug through hydrogen bonding and π-π stacking. Then, the obtained Ce6-erastin was extensively characterized and its anti-tumor efficacy towards OTSCC was evaluated both in vitro and in vivo.
Results: SLC7A11 expression is found to be upregulated in OTSCC, which is a potential target for ferroptosis-mediated OTSCC treatment. Ce6-erastin nanoparticles exhibited low cytotoxicity to normal tissues. More significantly, The over-accumulated intracellular ROS, increased O2 concentration and inhibited SLC7A11 expression lead to enhanced toxicity to CAL-27 cells and satisfactory antitumor effects to xenograft tumour mouse model upon irradiation.
Conclusion: Our ferroptosis promoted PDT approach markedly enhances anticancer actions by relieving hypoxia and promoting ROS production, thereby our work provides a new approach for overcoming hypoxia-associated resistance of PDT in cancer treatment.
Keywords: photodynamic therapy, ferroptosis, Fenton reaction, carrier free, nanodrug
 Global reach, higher impact
Global reach, higher impact