13.3
Impact Factor
Theranostics 2019; 9(11):3308-3340. doi:10.7150/thno.33888 This issue Cite
Review
Precise cell behaviors manipulation through light-responsive nano-regulators: recent advance and perspective
1. Sino-Singapore International Joint Research Institute (SSIJRI), Guangzhou 510000, China.
2. Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore
3. International Nanobody Research Center of Guangxi, Guangxi Medical University, Nanning, Guangxi, 530021, China
Received 2019-2-7; Accepted 2019-4-8; Published 2019-5-18
Abstract
Nanotechnology-assisted spatiotemporal manipulation of biological events holds great promise in advancing the practice of precision medicine in healthcare systems. The progress in internal and/or external stimuli-responsive nanoplatforms for highly specific cellular regulations and theranostic controls offer potential clinical translations of the revolutionized nanomedicine. To successfully implement this new paradigm, the emerging light-responsive nanoregulators with unparalleled precise cell functions manipulation have gained intensive attention, providing UV-Vis light-triggered photocleavage or photoisomerization studies, as well as near-infrared (NIR) light-mediated deep-tissue applications for stimulating cellular signal cascades and treatment of mortal diseases. This review discusses current developments of light-activatable nanoplatforms for modulations of various cellular events including neuromodulations, stem cell monitoring, immunomanipulation, cancer therapy, and other biological target intervention. In summary, the propagation of light-controlled nanomedicine would place a bright prospect for future medicine.
1. Introduction
The interrogation of precise cellular event manipulation is currently highly demanded in specific biomedical applications [1]. This great interdisciplinary frontier has perceived abundant efforts in designing systems that are capable of regulating cell fates for theranostic innovations. Nevertheless, relevant clinical applications of these systems are still limited due to general side effects stemming from the insufficient accumulation of therapeutics agents, lack of tunable monitoring over amplitude, time and location at deep tissue level [2]. These observations drove the advancement of stimuli-responsive platforms which exploit endogenous stimuli (such as pH, enzyme and redox reactions, etc.) and exogenous stimuli (such as light, magnetic fields, ionizing irradiation, etc.) [3]. As a significantly orthogonal external stimulus, light represents a noninvasive mean to spatiotemporally modulate predestined cell functions [4]. Short-wavelength light (e.g., ultraviolet (UV) and visible light, etc.) has been intensively used for either cleaving the photolabile groups on the therapeutic molecules or activating photosensitive agents, thereby triggering biological activities in precise time and specific pathological regions [5]. Alternatively, light-responsive proteins have recently been utilized as effective regulators to reversibly and promptly dissect cell functions, protein interaction as well as gene expression [6]. These genetically encoded photosensitive protein systems are known as optogenetic tools, which are particularly exquisite models in high spatiotemporal resolution cell fate influencing [7].
Considerably, in comparison with the UV-Vis light, the “tissue transmittable window” near-infrared (NIR) light ranging from 700 to 1000 nm with less tissue scattering and absorption offers much deeper penetration depth and minimized photodamaging [8]. These optical properties are exceptionally beneficial in the current vast development of deep tissue theragnostic. Particularly, near-infrared light responsive nanomaterials have enabled opportunities in noninvasive remote control of signal-transduction cascades, cell behaviors and further extended to regenerative medicine and anticancer treatments [9-11]. These nanomaterials with large extinction coefficient in NIR region could enable the release of loaded therapeutics at a predetermined tissue or could play as optogenetic tools to stimulate the implanted optogenetic engineered cells by a NIR source located outside the body [12].
Benefiting from the advancements in light-responsive nanomaterials, neuroscience has been profoundly transformed with targeted photoreceptors expression in neurons for their selective activation and inhibition [13]. Similarly, noninvasive modulation of stem cells and immune cells have been empowered with accurate monitoring of their extracellular microenvironment or intracellular signaling cascades [14, 15]. Beside, ablation of cancer has been enhanced with the minimum off-target toxicity and reduced metastasis and recurrence [16]. Furthermore, these nanoplatforms could trigger multiple cellular activities including gene transcriptions, cell migrations and interactions and could be employed in many other disease treatments namely vision restorations, cardiopathy and diabetes [17, 18]. Undoubtedly, the development of this rapidly evolving field would offer a bright perspective on the future of human medicine.
This review summarizes the recent development in photo-responsive nanomaterials ranging from inorganic to organic self-assembling and biomimetic nanoparticles for controlled drug delivery, investigating the activation and suppression of cell behaviors. We then examine the applications of these nanoplatforms in various cell types specializing in neurons, stem, immune, cancer cells and some other biological targets. Finally, we present our assessments on current challenges and provide our perspective on next-generation light-mediated nanomedicine.
Illustration of light-responsive nanoparticles for diverse cell functions regulations and precision medicine.
2. Light-responsive nanomaterials
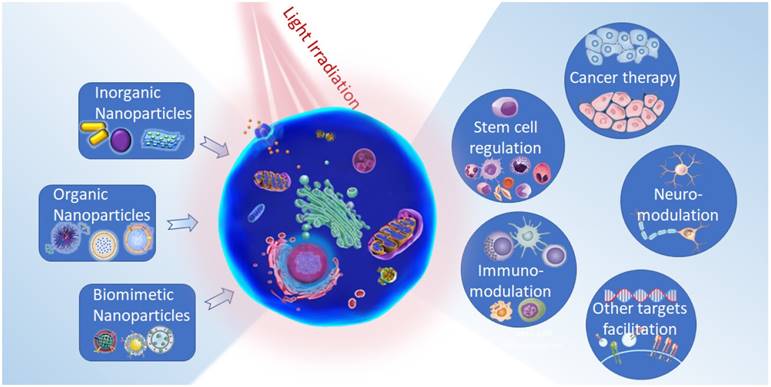
Light its own, depending on their wavelength and power density, possesses varied functions and features which in combination with suitable nanoparticles would provide multiple photo-triggered theranostic modalities. Light-sensitive multifunctional nanoparticles can be used in targeting to the specific location of interest and tracking by various imaging techniques, such as radiology and optical imaging. Using external light source excitation, these nanoparticles can then be used for the activation of on-called therapeutic agents that results in precise regulation or treatments. Generally, there are three types of photo-triggered theranostics namely photodynamic, photothermal and photoactivation of chemotherapeutics. Particularly, the photodynamic system relies on the activation of photosensitizers (PS) to induce the formation of reactive oxygen species (ROS) (e.g., peroxides, superoxide, hydroxyl radical, singlet oxygen (1O2)) that stimulate cellular mediations [19]. This system generally requires a relatively low laser power, but the sufficient amount of oxygen in interested areas is pivotal for its efficiency. Noticeably, most of PSs are hydrophobic molecules with poor water solubility that usually led to limited accumulation at the targeted regions. This issue might cause off-target photochemistry reactions which are harmful to healthy and nontargeted cells. It is also important to note that a major number of PSs are activated only by UV/Vis light, which may have potential concerns of light toxicity and limited tissue penetration [20]. Differently, photothermal modulation systems utilize photo-absorbers to convert light photon energy to heat. The controllable localized temperature increase can be employed for monitoring different heat sensing systems in the cell and thereby regulating the cellular processes [21]. For applications of this modality, several difficulties such as high laser power requirement and possible induction of overheating, inflammation effects should be noticed. Regarding photoactivation systems, light irradiation induces chemical structure conversions resulting in the cleaving of photosensitive functional groups that block the activities of caged molecules or the isomerization and conformational exchange of moieties. In the last decades, various photocaged molecules, such as o-nitrobenzyl (ONB), pyrenylmethyl ester, coumarinyl ester, and photoisomerization moieties, including azobenzene, spiropyran, and diarylethene have been established for spatiotemporally regulating biomolecular activities, real-time monitoring of cell trafficking and controlled release of therapeutic molecules or ions (as in case of photosensitive ion channels) in vitro and in vivo [22]. With assistance from nanoparticles, delivery of photosensitizers, photo-absorbers, and photoactivatable small molecules can overcome major limitations of their hydrophobicity, nonspecific accumulation and targeting. In integrating these modalities into nanotechnology, it should be acknowledged that each model possesses different strengths and their performance efficiency largely depend on the designs and purposes of the targeted cell functions.
In the recent era of advancement in various light-based biomedical applications, development of effective light responsive nanoplatforms is one of the essential prerequisites. The intrinsic characteristics of these materials regarding optical functions and physicochemical properties enormously influence the innovation of precision nanomedicine. Base on that view, photonic nanomaterials can be classified into three main types consisting of inorganic, organic and biomimetic nanoparticles. In addition, the assemblies and the hybrid nanostructures constructed from these nanoparticles would bring profound beneficial for versatile cellular functions monitoring.
2.1. Inorganic nanoparticles
Inorganic materials play an integral part in biology and medical science which are varied basing on abundant element constituents with diverse functionalities. Generally, inorganic photosensitive nanoparticles such as semiconductor NPs and quantum dots indicate narrow and tunable emission spectrum with minimal photobleaching. Comparing with other types of NP, inorganic NPs represent higher stability and significant light converting efficiency regarding photothermal aspect [23]. Some materials have been very widely used such as silicon dioxide (silica) which is versatile and relatively inert. Silica can be synthesized with nanometer-scale pores (e.g. mesoporous silica) that can be used to encapsulate other materials or cargoes, and the surface can be conjugated using silane chemistry. Recent developments in this type of materials have greatly promoted the research towards the applications of light-activated nanoplatforms, mainly attributed to their mature synthesis technology and unique pores structure properties [24, 25]. One of the representative light-responsive inorganic nanostructures, upconversion nanoparticles (UCNPs), which can absorb photons of NIR light irradiations to emit multiple shorter wavelength light (UV or visible), have recently received considerable attention. Such unique property is significantly attractive for bioimaging and nanomedicine. Compare to other candidates, UCNPs offer a variety of advantages such as massive anti-Stokes shifts, sharp emissions, long luminescence lifetimes, and high resistance to photobleaching. Outstandingly, this kind of nanomaterial provides lower photodamage effect and higher penetration depth which are important for in vivo applications [26]. The recent advances in fabricating UCNPs mainly depend on surface passivation method providing core-shell nanostructures with enhanced photo-conversion efficiency. Notably, significant luminescence increase was obtained by conducting core-shell-shell structure with optimized content of ytterbium ion (Yb3+) [27] or designing a new type of core-shell UCNPs (NaYbF4: Tm@NaYF4) instead of conventional NaYF4: Yb/Tm@NaYF4 UCNPs [28]. In order to shift the excitation band to the biological window of around 800 nm, Nd3+ ions are customarily added as a sensitizer [29]. Additionally, some NIR fluorophores can be used for tuning UCNPs excitation bands ranging from 780 nm to 850 nm [30]. Regarding their biological applications, combinations of UCNPs with other light-sensitive opsins can greatly broaden the avenue for the noninvasive NIR control of biological processes in a highly precise manner [31].
Another representative inorganic material is gold nanoparticles (AuNPs) which are capable of offering a unique surface plasmon resonance-enhanced light absorbance and scattering ability. These nanoparticles not only serve as an attractive candidate for fluorescence enhancing/quenching, surface enhanced Raman spectroscopy (SERS), and photoacoustic imaging, but can also act as highly localized heat source for photothermal conversion efficiency [32]. Notably, the wavelength of light absorbance can be easily adjusted by modifying the gold nanoparticles shapes. Toward this end, various kinds of Au nanocarriers such as nanorods, nanostars, nanoshells, Au-shelled nanocomposites have been fabricated for diverse biological applications ranging from photothermal therapy to photothermal regulation of specific cell functions [33-35].
Other important inorganic nanoplatforms including ZnO, TiO2, CdSe/ZnS quantum dots, Cu2S nanoparticles, WS2 and MoOx nanosheets, RGO (reduced graphene oxide) nanosheets, SWNTs (single-walled carbon nanotubes) have also performed their importance in suppling either reactive oxygen species (ROSs) for photodynamic activations or inducing significant photothermal effects for important light-controlled medicines [36-39].
Despite their well establishment and multiple applications, inorganic nanoplatforms are still facing several drawbacks regarding the low biodegradable and possible long-term toxicity [40, 41]. For instance, QDs have been reported to be toxic due to the components of core nanocrystal material, the size of the QDs and some other factors such as dose and the presence of capping materials. In addition, extensive studies also indicated that carbon-nanotube (CNT) could potentially cause detrimental side effects to living cells including damaging cell envelope, interference with transmembrane electron transfer, generation of ROS and oxidization of cellular components as well as induction of inflammation and possible effects on carcinogenesis [42]. As for AuNPs, even though they have been considered as less toxic with negligible effects on cellular functions, several side effects have been indicated such as reduced cell viability dependent with dosage, formation of DNA adducts, oxidative stress, and lactate dehydrogenase leakage [43]. For UCNPs, although insignificant toxicity has been reported [44], the long-term toxicity investigations are still required since their nondegradable and highly stable nanocrystals might interfere with the activities of other cellular components in living systems.
2.2. Organic nanoparticles
Organic photosensitive agents are one major player in the light-activating platform for applications in biological systems. Under light illumination, these agents absorb photons to elevate electrons from its ground state to a higher excitation state, the molecules are then placed in the lowest vibrational level through internal relaxation. Further relaxation of the agents could then induce the emission of a photon with a stock shift in wavelength; the transfer of energy in heat form; or the conversion from singlet to the triplet state, follow by photon emission or generation of ROS or 1O2 [45]. Despite their light-controlled efficacy and biocompatibility, most small molecule agents suffer from poor pharmacokinetic characteristics. To address these problems, multiple functional organic groups, polymers or organic self-assembling nanoplatforms have been investigated for their light-responsive applications. These organic nanoparticles, also defined as assemblies of carbon-containing macromolecules, can be tailored to design rational structure for varied requirements of intended applications and avoiding photobleaching suffered in small molecule organic dyes [46]. Such flexibility, coming from noncovalent intermolecular interactions, offers high biodegradability and unique ability to change morphology and conformation in response to environmental stimuli. Under light activation together with the modifications of pH, redox reactions, enzyme or homeostatic interactions, the environment-responsive nanosystems would significantly enhance the possibility of precise medicinal applications and reduced off-target toxicity [47]. Furthermore, the interaction of chromophores with organic NPs might lead to unique optical properties that expand the purview of photonic techniques. Taking these advantages in consideration, various forms of organic NPs have been fabricated. For instance, light responsive polymers which conjugate different light-responsive photochromic moieties as azobenzene, coumarin, spiropyran O-nitrobenzyl (ONB), 4-bromo-7-hydroxycoumarin (BHC), and 2-diazo-1,2-naphthoquinone (DNQ) in organic polymer structures have promoted the photoinduced transformations and photocleavage reactions [48-52]. The organic polymers could also encapsulate photo-responsive molecules like chlorine e6 (Ce6), Br-substituted boron-dipyrromethene (BODIPY) or metal complexes for good colloidal stability and controlled photodynamic therapy [53-55].
Currently, there are various photosensitizer molecules (PSs) have been developed which include porphyrin-, cyanine-, and polymer-based dyes. Typically, porphyrin-based PSs, a class of heterocyclic rings containing organic molecules (such as porphyrin, chlorine, bacteriochlorin, etc.), can emit strong fluorescence and generate remarkable 1O2 upon light irradiation. Assembling porphyrin-based PSs into nanostructures results in hydrophobic collapse, π-π stacking between PS moieties which greatly improves their pharmacokinetic properties and treatment effects for photodynamic therapy. Toward this direction, porphysomes (a type of new porphyrin-lipid nanovesicles) have been constructed by self-assembling or co-assembling of porphyrin-based PSs phospholipids. Noticeably, porphysomes demonstrate the extended half-life photodynamic [56] and high thermal conversion efficiency [57]. Differently, porphyrin-based PSs could be engineered to micelle-like particles by conjugating with biopolymers like peptide, polysaccharides, or functionalized with other NPs for multifunctional purposes [58]. Alternatively, cyanine dyes are other choices for photoactivated organic PSs. These dyes can be characterized by the polymethine functional groups with their fluorescence properties been intensively studied. Indocyanine green (ICG), a representative FDA approved fluorescent imaging contrast agents, has been extensively applied in clinics with its significant absorbance at NIR window (λmax at around 800 nm). Besides the promising utilization in molecular imaging, ICG-based nanomaterials have been proved to enhance biosafety and to generate profound heat for photothermal effects [59]. On the other hand, polymers containing multiple aromatic rings such as polypyrrole (PPy), polyaniline (PANI), polydopamine (PDA), poly(3,4-ethylenedioxythiophene): poly(4-styrenesulfonate) (PEDOT:PSS), which have strong light absorption, can thus provide excellent heat effects for photothermal functions [60-62]. For example, the photothermal efficiency of PPY and PANI have been reported to be approximately 44.7% and 48.5% respectively, which are much higher than the photothermal effect from the popularly utilized gold nanorods (21%) [60, 61].
Usually, organic NPs exhibit higher biocompatibility as well as minimum cytotoxicity and proinflammatory effects in cellular systems. However, cautious evaluations should be processed since in vitro toxicity may significantly differ from in vivo performance. ICG, for example, its free molecules can be easily cleared from the body while the retention time of ICG-based NPs would be much prolonged. Therefore, the long-term toxicity should be taken into serious consideration. Besides, other organic NPs such as cationic liposomes can sometimes cause genotoxicity, inflammatory responses, as well as oxidative stress and DNA damage [63]. Hence, rational designs of organic nanostructures with sufficient photoactivated responses as well as thorough consideration of side effects are indispensable for the clinical translations.
2.3. Biomimetic nanoparticles
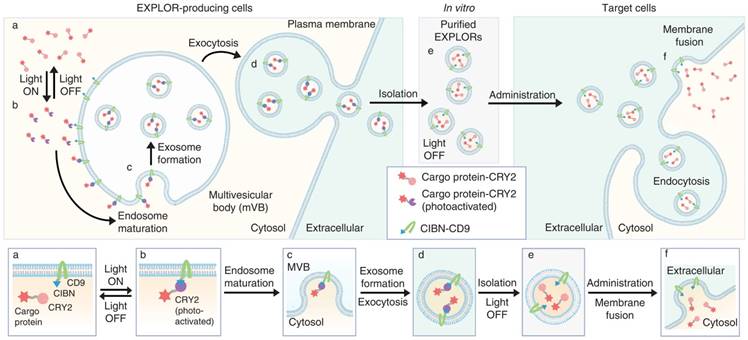
Recently, the dynamic transportation of nanoparticles in the vascular system, their clearance or phagocytic immune-mediated degradation promotes a significant concern toward designing nanoplatforms with specific biological functionalities. Therefore, different inorganic and organic hybrid nanostructures have been integrated with various unique bio moieties including lipids, glycans, and other cell membrane components. Among these, membrane-derived nanocomplexes have shown their prominent advantages including evading macrophage uptake, avoiding immune clearance, and enhancing circulation time in blood. Typically, these nanoplatforms are derived from natural host cells membrane (e.g., erythrocytes, leukocytes, platelets, stem cells, etc.) or invasive pathogens (e.g., bacteria, viruses, etc.) [64-68] in integration with the predetermined inorganic or organic nanocores. These nanocores such as AuNPs, UCNPs, polymeric nano-assembles are responsible for the light-activatable properties of the whole nanosystems which have gained intensive interests recently [67, 69, 70]. Other biologically self-assembled nanoparticles with promising unique characteristics such as fusogenic liposomes, exosomes have also been exploited as nanocarriers for direct cytosolic delivery of therapeutic agents or proteins [71-73]. In particular, the natural cell-derived vesicles like exosome, which originated from internal endocytic compartments and multi-vesicular bodies, can involve in intercellular communication by transporting macromolecules from one to another. Inspiringly, these exquisite nanoplatforms can be used for delivery of proteins, photosensitive molecules or photoactivation channels which are fruitful for precise regulation of targeted cell functions [74, 75]. Furthermore, these transportations could be monitored with light irradiation by combining with the optogenetic tools as illustrated in Figure 1. In this system, a photoreceptor cryptochrome 2 (CRY2), and CRY-interacting basic-helix-loophelix 1 (CIB1) protein module could be selected for controllable, reversible loading and delivery of proteins into exosome. The truncated version of CIB1, CIBN, was conjugated with an exosome-associated tetraspanin CD9. Under blue light excitation, CRY2-conjugated cargo proteins docked with CIBN and therefore being stabilized on the exosome membrane. Once switching off the illumination source, the proteins were able to release from CD9-conjugated CIBN and were efficiently delivered into the cytosolic compartment of target cells.
Additionally, bacterial outer membrane vesicles (OMVs) can also be produced from bacteria. These nano-vesicles show several advantages as they offer rigid membrane which attains high stability and reduce leakage in the systemic circulation. Importantly, since bacteria can easily be modified genetically to produce desired agents, OMVs can be thus customized to carry desired payloads which are useful in vaccination, bio-sensing and imaging, as well as targeted delivery. Furthermore, it can be scaled up using fermentation and optimized purification procedures. Recently, some bacteria were engineered to express one typical rate-limiting enzyme in melanin biosynthesis, Rhizobium etli tyrosinase. By this protocol, melanin would be produced and accumulated in the bacterial cytosol and periplasmic space, and then it could be encapsulated within membrane and cytosol of OMVs. Considering that melanin can be found naturally in many living organisms and it absorbs strongly in the visible and NIR window, which is therefore well suited for usage as contrast agents in optoacoustic imaging and photothermal therapy. Latest results clearly illustrated that this OMVs encapsulating biopolymer-melanin generates the strong optoacoustic signal and significant photothermal effect both in vitro and in vivo under NIR light irradiation [76]. This work imparts new insight of bioengineered vesicles as potent alternatives to synthetic particles for light-controlled diagnostic and therapy.
Although possessing multiple advantages, some potential shortcomings still need to be further solved in these biomimetic nanoplatforms. One obvious drawback is the complicated preparation method associated with insufficient quantity, low stability and integrality level. Extensive modification processes such as genetic engineering could compromise or alter the original entities [77]. Moreover, the membrane components like glycan or proteins could be malfunctioned during fabrication periods and could induce undesired safety effects including immunogenicity [78]. Particularly, exosome-based systems show low stability which usually demand complicated, expensive purification and production strategies. Erythrocyte-derived system frequently lacks a nucleus that inhibits them from genetically engineering to carry biologically derived cargos. Moreover, yeast vacuoles show good tissue penetration ability and easy to scale up, but their long-term stability, possible immunogenicity, and toxicity may raise consistent concerns. Therefore, significant optimizations of these nanocomplexes are still highly desirable before advancing to meet clinical and commercial needs.
Schematic illustration of exosomes for protein loading via optically reversible protein-protein interactions. (Adapted with permission from [75], copyright 2016 Nature Publishing Group)
Inorganic, organic or biomimetic nanoplatforms have showed their different strong points and drawbacks. The incorporation of those types together for minimizing individual weaknesses while complementing other advantages, is recently an emerging direction [79] for improving the efficiency of future nanotheranostics. By integrating two modalities of photodynamic and photothermal activation into a hybrid NP, for example, the efficiency of phototherapy would be elevated. Synergistic effects of these two modalities have been reported by an abundance of works recently [80]. Similarly, other synergistic bimodal and trimodal systems that incorporating photothermal, photodynamic and photoimmunotherapy have been significantly investigated as well. For example, IR 780 dyes could be encapsulated in an imatinib-loaded poly(lactic-co-glycolic acid) (GITR-PLGA) nanoparticles with the structure containing glucocorticoid-induced TNF receptor family-related protein. NIR light exposure of these nanostructures could trigger photothermal and photodynamic effects by IR 780 as well as promote immune responses. Together with the effects resulted from imatinib activation, the extraordinary activities to efficiently eradicate tumor growth, diminish tumor recurrence and improve survival in vivo can easily be realized [81].
Nanoplatforms capable of inducing multifunctional effects can also be fabricated by unique hybridization of distinct types of NPs including inorganic, organic and biomimetic NPs. For instance, the robust photothermal polydopamine coated spiky AuNPs have shown their remarkable improvement in photothermal efficiency in vitro and in vivo. Strikingly, subsequent treatment with a dose of antitumor drug, Doxorubicin (DOX), after PTT could elicit the anti-tumor response of CD8+ T and NK cells for eliminating residual tumor cells at local or distant tumor areas [82]. Moreover, polydopamine coated UCNPs also illustrated great potential and could work as useful multimodal imaging contrast agents for synergistic photo-activation in vitro and in vivo. Significantly, multiple functions including upconversion luminescence imaging, T1-weighted magnetic resonance imaging, X-ray computed tomography imaging, as well as PTT, and chemotherapy can be provided by one single united Doxorubicin-loaded upconversion @ polydopamine nanoprobe (UCNP@PDA5-PEG-DOX) [83].
Furthermore, upon combining of nanoparticles with different properties, the assemblies of NPs demonstrate diverse geometries and chemical characteristics that are applicable for sensitive detection and precise manipulation of cellular processes [84]. For instance, chiral nanodevices constructed from yolk-shell nanoparticles tetrahedron (UYTe), centralized with upconversion nanoparticles (UCNPs), were used to elicit autophagy in vivo. The chirality of the nanodevices were further tailored by modifying with different enantiomers of glutathione (GSH). Due to enhanced ROS generation and accumulation in living cells, UYTe was found to generate a chirality-dependent autophagy-inducing activity. Meanwhile, the disassembly of UCNPs and UYTe triggered by autophagy activation could reduce intracellular CD signal and produce more ATP which simultaneously induce an obvious signal increase in the UCL intensity [85]. Promisingly, this study demonstrates the tremendous potential utility of chiral nanostructures in cellular biology applications.
Extensive studies have clearly approved that chiral structures could trigger profound catalytic activity upon circularly polarized light (CPL) irradiation, and they could also be activated under CPL with a laser of all polarized angles compared with linear polarization light. The dependence on circular dichroism (CD) spectra could potentially allow the differentiation of the extracellular and intracellular localization of plasmonic assemblies. Recently, some DNA-driven shell-satellite gold assemblies were modified with cysteine enantiomers on their surface to provide chiral plasmonic nanostructures. Under irradiation of CPL, these chiral nanoassemblies could exhibit high ROS release which thereby represents a new avenue for theranostic applications using chiral nanostructures as multifunctional platforms [86]. Likewise, various chiral-nanoassembies fabricated from different inorganic NPs through DNA strands have been utilized for monitoring of multiple cellular functions and bioactive molecules including glycoproteins, micro RNA, and amino acids in living cells [87].
Rational integration of the process of light irradiation into multifunctional nanosystems is promising for a major improvement in transport, tracking, monitoring phototherapeutic agents and modulating various cellular functions. So far, intensive investigations of light-responsive nanoparticles have opened a bright future for the pre-clinical translation of nanomedicine. Undoubtedly, there are still abundant rooms for innovative breakthroughs and discoveries in this dynamic and fast-moving field.
3. Biological applications of light-responsive nanomaterials
3.1. Neuromodulation
In neuroscience, the stimulation of action potential represents a fundamental method for investigating the nervous systems and developing clinical treatments of neurological diseases [88]. Conventionally, the electric-based method has been proposed to electrically promote neuronal activities [89]. Basically, surgical placement of electrodes has been applied for manipulation of neuron action potential. However, limitations coming from the long-term biocompatibility and serious surgery trauma would hamper its applications in clinics [90]. Considerably, neural stimulation techniques with minimal invasion and significant spatial resolution are highly demanded in the clinical translation of neuroscience studies. Followed by this principle, light has been exploited with an additional assistance from nanomaterials to modulate electrical change in the neuronal system. Two most exceptionally beneficial models are optogenetics and optothermal neuromodulations.
3.1.1. Optogenetic neuromodulation
The introduction of light-activated proteins that can stimulate or suppress neuronal activities is one of the groundbreaking innovations in neuroscience in recent years. Combining advantages from genetic engineering and optical properties, this optogenetic method has become a promising approach that can offer high spatiotemporal control of neuronal activities with minimum invasion. Such an integration supplies an excellent strategy for real-time neuromodulation and treatment of neuronal disorders like Alzheimer's diseases [91-93].
Progress in optogenetic neuromodulation has perceived the evolution of light-gated ion channels with extended excitation bands, enabling both stimulation and inhibition of neuronal activities [94, 95]. Furthermore, optimization of other principle components including light delivery systems, activation sensors, and optogene delivery protocols have gained intensive attention. Particularly, due to the complexity of mammalian skull which minimizes the penetration of external light, integration of light sources into the brain tissue is necessary. Therefore, implantation of head-mounted LED [96], thin optical fiber [97] or wireless LED devices [98] have been performed which renders controllable regulation of neuronal activities in live animals. Nevertheless, invasiveness and low spatial manipulation typically narrowed the clinical applications of these devices. Alternatively, light-responsive nanomaterials have been employed as nanotransducers for optogenetics which could absorb excitation to produce visible light for modulating light-gated ion channels. One particularly powerful nanotransducer is UCNP which can be excited under NIR offering less scattering and deeper penetration depth.
In 2013, Lee's group demonstrated the implementation of UCNPs in neuromodulation. In this study, channelrhodopsin-2 (ChR2) in engineered neurons was stimulated by 980 nm laser excitable UCNPs that embedded on poly(lactic-co-glycolic acid) (PLGA) films with thickness of 0.5 µm. Upon NIR light pulses, the ChR2-expressed neurons generated time-locked, sustained naturalistic impulses with millisecond resolution [99]. Other groups have also contributed to this field using various UCNPs for activation of different channels [100, 101]. For example, Han and coworkers synthesized IR806 -sensitized UCNPs to manipulate Red-activatable Channelrhodopsin (ReaChR) using 800 nm laser excitation. This dye-sensitized UCNPs were loaded into poly(methyl methacrylate) (PMMA) polymers to provide thin films for hippocampal neurons culturing. They found that neurons could be spatiotemporally activated under potential firing in light-intensity dependent manner [102]. In addition, multiplexed NaYF4:Sc/Yb/Er@NaYF4 UCNPs that emit green light (550 nm) upon 976 nm exposure were used to monitor C1V1 or mVChR1 to generate a photocurrent in the neuron cells [100]. Similarly, NaYF4:Sc/Yb/Tm@NaYF4 UCNPs with blue emission were exploited to produce photocurrent in PsChR ion channel expressed cells.
Very recently, Thomas' group fabricated UCNPs with blue- or green-emitting to specifically match the action spectra of ChR2 and archaerhodopsin (Arch), respectively [103]. (Figure 2). UCNPs were then directly injected into the brain area of interest including a deeply situated ventral tegmental area (VTA) or hippocampal (HIP) (Figure 2B-E). It is important to note that even at a depth of 4.5 millimeters, UCNPs still generated enough short wavelength light upon irradiated by 980 nm NIR light to activate ChR2 or Arch. By using a viral vector in combination with Cre recombinase line, ChR2 was targeted to dopamine neuron in VTA. After exposing to a transcranial NIR stimulation, the neuronal excitation of ChR2 transfected mice was observed via higher expression of c-Fos and dopamine release. Significantly, UCNP-mediated optogenetic was applied for mediating behaviour of awaked animal by labelling active c-Fos-expressing dentate gyrus (DG) granule cells with ChR2, and by injecting blue-emitted UCNPs into DG region of c-fos-tTA transgenic mice before applying transcranial NIR stimulation. The results clearly showed the behaviour freezing of mice during light irradiation with the long-term in vivo utility.
UCNP-mediated NIR upconversion optogenetics for deep brain stimulation. (A) Schematic design of a blue-emitting NaYF4:Yb/Tm@SiO2 particle for regulation of ChR2. (B) In vivo experimental scheme for transcranial NIR stimulation of the VTA in anesthetized mice. (C) Confocal images of the VTA after transcranial NIR stimulation under different conditions. (D) Cumulative DA release within 15 s after the start of transcranial stimulation. (E) Schematic of a green-emitting NaYF4:Yb/Er@SiO2 particle and Illustration of transcranial NIR inhibition of hippocampal (HIP) activity during chemically induced seizure. (F) Confocal images of the hippocampus following transcranial NIR stimulation under different conditions. (G) c-Fos expression under the four conditions presented in (F) ( Adapted with permission from [103], copyright 2018 American Association for the Advancement of Science).
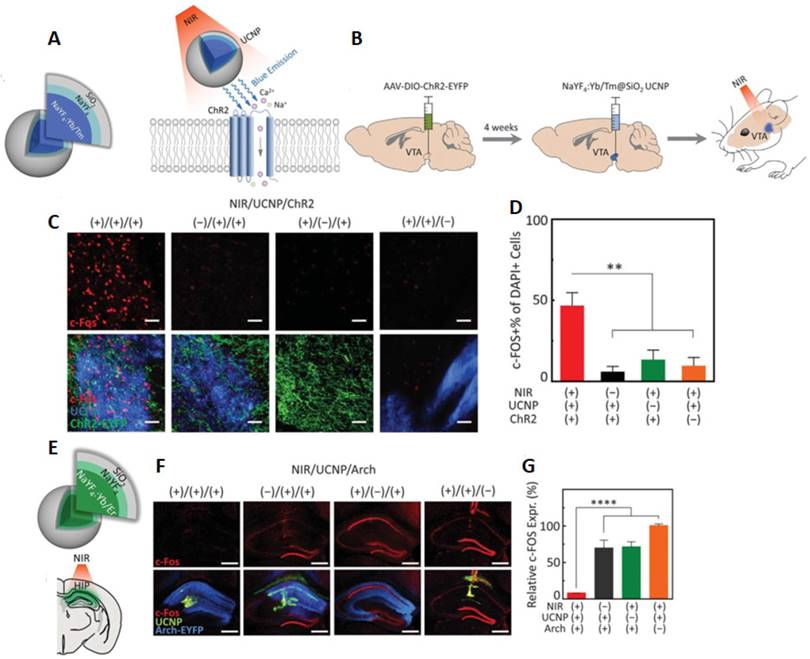
Noticeably, neuronal inhibition requires a relatively higher optical power in comparison with neural excitation. Therefore, a significant effort has been made by Lin's group to enhance the intensity of visible light generated from UCNPs for effective optogenetic inhibition. In this work, an optimized doping ratio of Yb3+ in NaYF4@NaYF4:Yb/Er core-shell-shell nanoparticles for enhanced 550 nm upconversion emission was performed. After embedding in a glass micropipette to fabricate an implantable upconversion device, the UCNPs were then able to place close to a targeted brain area which emitting a 550 nm light to explicitly control the enhanced natronomonas halorhodopsin (eNpHR) to inhibit neuron activity. The designed UCNP-optotrode devices showed their prominent of preventing direct contact of UCNPs with neuron as well as optimizing optical modulation by concentrating UCNPs in defined positions. Remarkably, these microdevices offered tetherless deep brain inhibition over free moving animals which renders promising future of remote behavior manipulation and its chronic applications [27].
3.1.2. Optothermal neuromodulation
Optothermal regulations of neural activities generally rely on the utilization of optical absorbers to convert light to heat, generating transient temperature rise in specific neuron area [104]. Such alteration of local temperature mediates the action potential propagation through modulating electrical capacitance of the plasma membrane or activates temperature-gated ion channels such as members of receptor potential vanilloid channel (TRPV) family [105, 106]. In order to reduce the off-target detrimental effects of excessive heat, nanotechnology has been exploited as photothermal nanotransducers to produce rapid temperature increase at a localized area. Toward this end, different photothermal nanomaterials have been proposed such as semiconducting or gold nanoparticles, mainly focusing on the utilization of NIR light to bring about photon-to-heat energy conversion. Such nanomaterials can be targeted to the plasma membrane, allowing precise opto-thermal manipulations of ion channels with minimal tissue damage by excessive heating [107, 108].
Plasmonic gold colloids have been used as an optothermal regulation of neuron by several groups so far [108-110]. Upon light irradiation, heat can be dissipated from gold nanoparticles through oscillation and collision of electrons on its surfaces [111]. Exploiting this intrinsic property, gold nanorods were illuminated by a 780 nm laser to activate nearby neurons in a study by Yong et al. [112]. Differently, this laser was used to promote Ca2+ influx in NG108-15 cells and stimulate neurite outgrowth through cellular internalized gold nanorods [113]. Recently, gold nanorods were functionalized with high-density lipoproteins in cationic form for targeted cell membrane interaction. Subsequently, upon photo-stimulation, the DRG neurons were modulated by activating the TRPV1 channel and monitoring of Ca2+ influx [114]. Noticeably, membrane-bound gold nanorods were also used as extrinsic absorbers to inhibit action potential as described by Yoo's group [115]. In this study, gold nanorods were functionalized with polyethylene glycol (NH2-PEG) to enable the interaction with the plasma membrane. Upon localized membrane photothermal effect, the TREK-1 thermosensitive potassium channels were activated causing inhibition of action potential. In addition, NP-assisted localized optical stimulation (NALOS) which follows a cell-targeting strategy to carry out photo-stimulations of subcellular regions on cultured hippocampal neurons was developed by Flavie et al. in 2016 [116]. In this study, plasmonic excitations of gold nanoparticles (AuNPs) upon 800 nm laser irradiation were able to trigger local Ca2+ transients, Ca2+ signaling and promote action potential via CaMKII in dendritic domains.
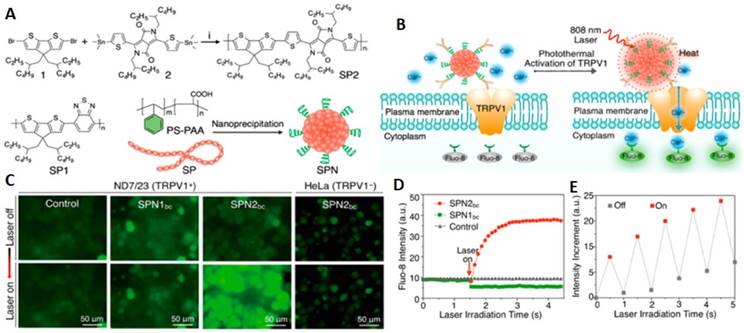
Furthermore, selective and rapid activation of temperature-sensitive ion channel TRPV1 can be achieved by using semiconducting polymer nanoconjugates (SPNs) as illustrated by a study of Lyu and collaborators [117]. This high biocompatibility nanomaterials circumvented heavy metal ion-induce toxicity and showed an excellent photothermal conversion efficiency which is a pivotal element in temporal control of input-output interrogation of neuron cells. For highly specific modulation of TRPV1 ion channels, SPNs were conjugated with anti TPRV1 antibody to become SPNsbc that performed extraordinarily in minimizing irradiation time and reducing off-target toxicity. Upon NIR laser irradiation at 808 nm, this targeted remote nanomodulators induce intracellular Ca2+ influx within milliseconds in a reversible manner. (Figure 3)
NIR photothermal activation of the TRPV1 ion channels in mouse neuroblastoma/DRG neuron hybrid ND7/23 cells. (A) Synthesis and characterization of SPNs. (B) Schematic illustration of SPNbc controlled photothermal activation of Ca2+ channels in neurons. (C) Fluorescence images of ND7/23 or HeLa treated with SPN1bc or SPN2bc before and after laser irradiation time at 808 nm (104 μWμm-2) for 2 s. (D) The fluorescence intensity of Fluo-8 as a function of laser irradiation time. (E) Changes in the fluorescence intensity of Fluo-8 with the laser irradiation at 808 nm switching on and off at an interval of 0.5 s (Adapted with permission from [117], copyright 2018 American Chemical Society).
In general, tremendous advances have been made on the nanoparticle-assisted optothermal and optogenetic neuromodulations. These strategies have not only revolutionized the study of neuronal circuitry but also indicate great potential in facilitating innovative neuronal stimulation schemes for the treatment of brain diseases. As powerful enabling tools, nanomaterials can be used in combination with other techniques including synthetic biology for dissecting neuronal functions. Inspiringly, there are still many unexplored avenues of optogenetic and optothermal approaches applying in neural circuits. Specifically, many essential tools and efficient targeting strategies remained to be developed. Current existing tools for inhibition of neural activity mainly required high light power intensity and hence induces considerable side effects [118]. Studies on optogenetic inhibition at the presynaptic terminal by using halorhodopsin, archaerhodopsin and chloride-conducting channelrhodopsins demonstrated that activation of light-gated chloride channels induced neurotransmitter release, constraining the application of these tools in temporal manipulation of synaptic release [118]. Therefore, complement studies on ion channel and other approaches for modulating neuronal cells will be highly demanded. For example, other than ion channels for triggering intracellular Ca2+-dependent signalling events, alternative methods such as optogenetic Ca2+ uncaging [119], light-gated Ca2+ release from the endoplasmic reticulum (ER) [120] should also be paid more attention. Alternatively, other important second messengers such as the local activity of downstream protein kinases, phosphodiesterases, nucleotide exchange factors can be regulated by light-activated phosphodiesterase, and numerous genetically encoded cAMP and cGMP sensors [121]. Light-responsive nanoparticles can therefore be used, targeting these molecules for regulation of neuronal differentiation and synaptic transmission. Furthermore, specific subcellular targeting of photo-responsive nanoparticles for studying of different neurons such as astrocytes, oligodendrocytes, microglia, and brain-resident stem cells and probing the strength of individual synapses are important for precise monitoring localized signalling events. For instance, Ca2+ in axonal boutons would be specifically facilitated by using presynaptic Ca2+ selective ChR [122]. Promisingly, with the emergence of abundance subcellular targeting motifs, various pathophysiology of the brain through light-responsive nanotechnology will be comprehensively investigated in the near future.
3.2. Stem cell regulation
Stem cells are unspecialized pluripotent cells possessing self-renewal capacities which can differentiate into different specific lineages given certain stimulus condition [123]. Generally, they are classified into three main types: embryonic stem cells (ESCs); adult stem cells (ASCs) (also known as somatic stem cells or tissue stem cells); induced pluripotent stem cells (iPSCs). Of note, a mesenchymal stem cell (MSC) is a type of adult stem cells which present in multiple tissues including umbilical cord, bone marrow, as well as fat tissue, and can be differentiated into multiple tissues such as bone, cartilage, muscle, fat cell and connective tissue [124]. This type of multipotent cells gained significant interest since they are easier to isolate, handle and differentiate than the other stem cells which are therefore superior in the process of regenerative engineering and tissue generation.
In the human body, extracellular matrix (ECM) and several cues modulate cell-cell communication and the interactions of the cell with the surrounding fluids, which in turn monitor stem cell fates consisting of viability, proliferation and differentiation. Thus, scaffolds that mimic nature topography-related cues with similar chemical and physical properties including size, morphology, surface roughness represent noticeably powerful platforms for tissue engineering and regenerative medicine [125, 126]. With respect to this direction, various scaffolds and nanomaterials have been constructed to act as either stem cell culture matrixes or biophysical cues for stem cell differentiation in diverse lineages which possibly produce promising clinical implications for organ (e.g., liver, kidney, lung, etc.) transplantation, and treatment of cardiovascular, orthopaedic, neurological as well as autoimmune diseases [127, 128].
3.2.1. Biochemical cues delivery
Genetic materials or small molecules are of paramount importance in the therapeutic application of stem cells. To date, small molecules and RNA interference (RNAi) technology for sequence-specific suppression of gene expressions are used to regulate a variety of stem cell behaviours including pluripotency, differentiation, and reprogramming [129, 130]. SiRNA, for example, has been proved to effectively control neural stem cell differentiation after being delivered into the cytoplasm of targeted cells [131]. Together with small molecule dexamethasone (Dex), siRNA was incorporated in a CdTe/ZnS core/shell QDs to minimize the main bottleneck of poor cellular uptake, accelerated degradation of siRNAs in biological fluids as well as poor water solubility of Dex. The QDs were modified with Cys-Lys-Lys-Arg-Gly-Asp (CKKRGD) peptide and amino-beta-cyclodextrin (NH2 -β-CD) on the surface to yield the RGD-β-CD-QDs nanocarrier. They found that codelivery of Dex and siRNA by this nanocarrier enhanced osteogenesis differentiation of human mesenchymal stem cells (hMSCs). Importantly, binding of these nanocomplexes with hMSCs have shown its ability to long-term tracking of implanted hMSCs in animals which are vital for obtaining information to improve the efficacy and safety of stem cell therapy in clinical translation [132].
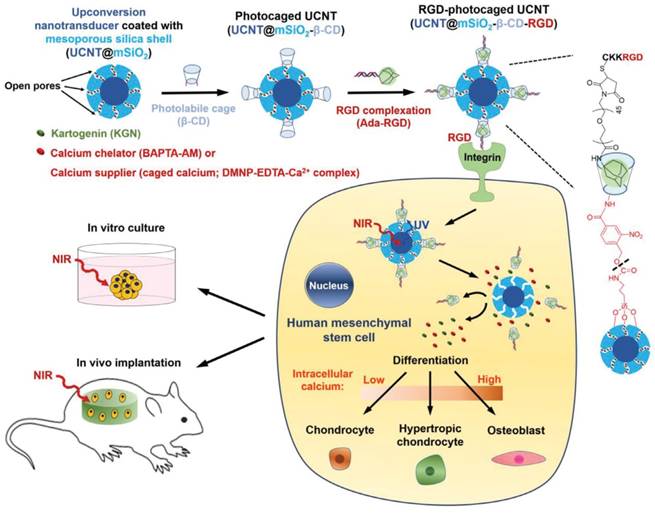
In addition, Kartogenin (KGN) have recently emerged as a nonprotein heterocyclic compound which induces chrondogenesis by binding with the actin-binding protein to dissociate core-binding factor β subunit (CBFβ). The released CBFβ subsequently translocated to the nucleus and forms a transcriptional complex with runt-related transcription factor RUNX1, which in turn promotes chondrocyte differentiation [133]. This small molecule was delivered into stem cells to enable the differentiation process by various nanoparticles [134-136]. For instance, KGN was delivered into specific stem cell in a stimuli-responsive manner by UCNPs. The study by Li's group demonstrated the design of multifunctional UCNPs with its remarkable uptake in hMSCs by RGD peptide conjugation which then cleaves the photocaged linker (4-(hydroxymethyl)-3-nitrobenzoic acid) to release KGN upon NIR trigger intracellularly. The in vitro and in vivo studies evaluated the low toxicity, deep tissue drug release for effective induction of stem cell differentiation and long-term tracking ability of the nanoparticles (even after 28 days) [137].
3.2.2. Biophysical cues stimulation
The effects of microenvironment cues on stem cell differentiation have been investigated to reveal a significant role of stress relaxation, substrate elasticity, degradation, and mechanical dosing in deciding the outcomes [138-140]. For example, multidirectional differentiation of MSCs was guided by a photocontrolled upconversion-based substrate [141]. 4-(hydroxymethyl)-3-nitrobenzoic acid modified poly(ethylene glycol) attached on the substrate could be released in laser power-dependent manner to monitor the level of cell-matrix interaction. By this strategy, MSCs on the substrate were facilitated to differentiate into adipocyte or osteoblast by 0.5 or 6 W/cm2, respectively. This work provides a piece of evidence that smart artificial interface materials can dynamically switch stem cell differentiation via an alteration of the microenvironment.
It is noteworthy that intracellular calcium level is also an important factor to determine the direction of stem cell differentiation [143, 144]. Recently, KGN was effectively delivered into hMSCs by UCNPs similar to the previous studies. Notably, calcium chelators (BAPTA-AM) or calcium suppliers (DMNP-EDTA-Ca2+ complex) were loaded into silica shell on UCNPs surface which later inducing fluctuation of intracellular Ca2+ level to regulate distinguished stem cells differentiation outcomes (Figure 4). Specifically, the release of BAPTA-AM down-regulated the Ca2+ level to induce classic chondrocyte, whereas, the introduction of calcium supplier into the cytoplasm of stem cell triggered its differentiation toward osteoblast. Meanwhile, the supply of KGN alone drove the hMSCs to hypertrophic chondrocyte. NIR irradiation used in this study not only manipulate the differentiation but also play an important role as a trackable material of this process in vitro and in vivo [142].
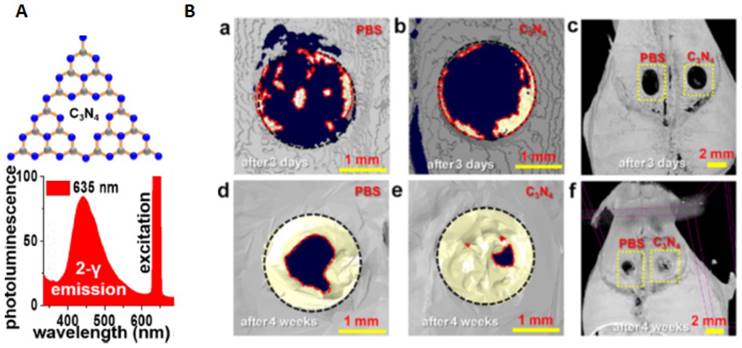
The Ca2+ level can also be regulated by red-light absorbing carbon nitride (C3N4) sheets, inducing human bone marrow-derived mesenchymal stem cells (hBMSCs) differentiation into osteoblast [145]. Upon the exposure of the red light, C3N4 sheets were excited by two-photon excitation resulting in electron release and generation of photocurrent in the nuclei and cytoplasm of hBMSCs inducing inter and intracellular charge forces. Accordingly, Ca2+ ions translocated into the stem cell via voltage-gated ion channels which then activated calmodulin, stimulated cellular proliferation and osteogenic differentiation. Significantly, in vivo bond generation in mouse cranial defects using red light activated C3N4 sheets was conducted demonstrating a remarkable augment new bone repair. Specifically, the restored area increased from 19.6 ± 2.0 % (after 3 days) to 91.1 ± 3.2 % after 4 weeks while the control with PBS treatment shown 69.7 ± 2.9 % recovery after 4 weeks (16.7 ± 1.1 % after 3 days) (Figure 5).
These studies clearly revealed that stem cells differentiation could be directly regulated by controlling intracellular calcium. Harnessing the current great advance in remote control of intracellular Ca2+ by light [146], this method would potentially accelerate the stem cell-based regenerative therapies and advance the treatment of complicated diseases.
3.3. Immunomodulation
3.3.1 Photoimmunotherapy
Immunomodulatory therapies are recently indisputably one of the leading edges in anticancer therapy. Currently, cancer immunotherapy is associated with on-target, off-tumor cytotoxicity or immune-related adverse events [147]. Thus, smarter immunotherapies with enhanced safety and precise control over the anticancer immune response are needed. In this situation, nanomaterials serve as either delivery platforms for targeted transport of immunostimulatory agents or the intrinsic immunomodulators for selective regulation of important signaling pathways within distinctive immune cell populations. With the purpose of enhancing precision in regulating the immune system for cancer or autoimmune treatments, light-responsive nanomodulators have attained significant interests.
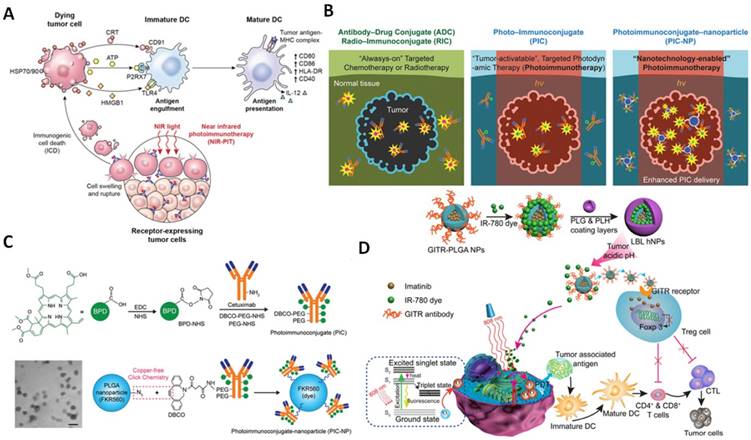
One particularly exciting avenue is the manipulation of immunogenic cell death (ICD) characterized by alterations in cell surface components and the release of soluble mediators [148, 149]. As pioneers of studies in this area, Zitvogel and Kroemer described ICD as the generation of immunogenic signals upon different stimuli such as heat shock protein (HSP), endoplasmic reticulum chaperone calreticulin, ATP and high mobility group box 1 (HMGB1) which are classified as damage-associated molecular patterns (DAMPs) [150]. These DAMPs trigger the maturation of dendritic cells (DCs) to stimulate the expression of tumor antigen to T cells (Figure 6A). This concept is a driving force for the emergence of near-infrared photoimmunotherapy (NIR-PIT) relying on photosensitizer-antibody photo-immunoconjugates (PICs) which target cell expressing antigens and after non-invasive NIR light exposure, it induces highly selective necrotic cell death without affecting adjacent healthy cells. Although many attempts of developing PICs have shown its promising prospects, the limited number of PSs to the cancer area is still a persistent challenge. A study by Huang et al. recently proved that nanotechnology could be used to overcome this obstacle (Figure 6B). By incorporating the PEGylated BPD-Cetuximab PIC system and biocompatible poly(lactic-co-glycolic acid) (PLGA) nanoparticles, they found that the obtained PIC nanoparticles (PIC-NPs) doubled intratumorally accumulation of PICs in comparison with normal immunoconjugates (Figure 6C). This improvement of carrier efficiency, thereby, enhanced photoimmunotherapy mediated tumor volume reduction [151]. Nanoparticle-based photoimmunotherapy has opened a broad avenue for generating tumor-associated antigens which in turn activates the immune system to prevent tumour recurrence and metastasis.
NIR-mediated control of photo-uncaging and intracellular release of KGN and either calcium chelator or calcium supplier (caged calcium) by UCNT-based nanocomplex for stem cell regulation (Adapted with permission from [142], copyright 2018 Wiley-VCH).
C3N4 sheets for the enhancement in repairing cranial bone defect under red light. (A) C3N4 sheets structure and the photoluminescence spectra. (B) Analysis of bone regeneration in critical size cranial defects under red light. (a-f) 3D μ-CT images after 3 days or 4 weeks of PBS and C3N4 sheet-assisted treatments. Regenerated bone is indicated with a yellow color. (Adapted with permission from [145], copyright 2017 American Chemical Society).
(A) The mechanism of NIR-PIT induced immunogenic cell death (Adapted with permission from [152], copyright 2017 Impact Journals). (B) photo-immunoconjugates nanoparticles for enhanced photoimmunotherapy efficiency. (C) Schematic depiction of PIC-NPs synthesis. Benzoporphyrin derivative (BPD) photosensitizers were conjugated to PEGylated Cetuximab via carbodiimide crosslinker chemistry, Azide-containing FKR560 dye-loaded PLGA nanoparticles were reacted with the dibenzocyclooctyne (DBCO)-containing PICs to form PIC-NPs (B and C were adapted with permission from [151], copyright 2018 Wiley-VCH). (D) Schematic illustration of NIR therapy and regulatory T cell modulation using layer-by-layer hybrid nanoparticles (LBL hNPs) with mutual PTT, PDT, and immune-anticancer therapeutic effects. (Adapted with permission from [81], copyright 2018 Ivyspring International Publisher).
It is noteworthy that tumor cells normally express programmed death-ligands (PDL1 and PDL2) while T cells produce cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein-1 (PD-1) to suppress the activation of CTLs [153]. Therefore, immune suppressive blockers are needed to treat tumor before the operation of photothermal or photodynamic therapies (PTT or PDT) [154, 155]. In line with this, researchers have combined nanoparticles with immunostimulatory drugs namely immunoadjuvant CpG oligodeoxynucleotides (CpG ODN) on graphite oxide (GO) or chitosan-coated Cu2S. By encapsulating CpG ODN in chitosan-coated Cu2S, the fabricated nanocomplexes after exposure to light activated DCs, NK cells and stimulated the proliferation of cytotoxic T lymphocytes [156]. Noticeably, three clinically approved ingredients including photothermal converter ICG, imiquimod (R837), a toll-like receptor 7 agonist were co-encapsulated together by poly lactic-co-glycolic acid (PLGA), providing a high potential clinical applicable nanoparticles [157]. Significantly, the checkpoint-blockade, anti-CTLA4, was employed to inhibit metastases. In fact, checkpoint inhibitors such as anti-CTLA-4 can increase the infiltration of T cells and reduce the immunosuppressive function of regulatory T cells in the tumor microenvironment. Applications of this effect have also revealed efficient immune responses after photoimmunotherapy in an earlier study that integrates SWNTs with anti-CTLA-4 antibody [158].
Significantly, photodynamic treatment can also trigger an antitumor immune response as demonstrated by various studies recently [159, 160]. For example, the photosensitizers chlorin e6 (Ce6) were co-loaded with imiquimod (R837), a Toll-like-receptor-7 agonist, onto UCNPs surface. The photodynamic activation by NIR light-responsive UCNP-Ce6-R837 nanoparticles was able to promote significant antitumor immune responses. Moreover, in combination with CTLA-4 checkpoint blockade, the nanoparticles provided a non-invasive tumor ablation method with a long-term immune memory function [161]. Recently, IR-780 and imatinib-loaded layer-by-layer hybrid nanoparticles were fabricated to promote simultaneous photothermal and photodynamic therapy upon endogenous release of IR780 in the low pH tumor microenvironment (Figure 6D). The direct cell apoptosis/necrosis promoted the presentation of tumor-associated antigens to T cells, while imatinib-loaded GITR-PLGA cores inhibited Foxp3 expression, impaired Treg immunosuppressive functions, consequently activated effective CD8+ T cells towards tumors [81].
3.3.2. Macrophage reprogramming
Tumor-associated macrophages (TAMs) are the critical drivers of the immunosuppressive tumor microenvironment, playing a pivotal role in cancer recurrence, invasion, angiogenesis, metastases. These immune cells are characterized as two distinct populations, classically activated pro-inflammatory (M1) phenotype (attacking invaders) and alternatively activated anti-inflammatory (M2) phenotype (healing damages) [162]. While M1-polarized macrophages secret cytotoxic cytokine (e.g., TNFα, IL-6) to inhibit post-treatment cell growth, M2-polarized macrophages promote tissue repair, immunosuppression and tumor remission. In fact, the M2-type TAM population typically overwhelms the number of M1-type in the tumor microenvironment which therefore increases the chance for tumor recurrence after treatment [163]. Intriguingly, M2 can be reprogrammed to become M1-polarized macrophages by monitoring physiological and pathological environments. Therapeutics that redirect M2 to M1 phenotype would alleviate not only the TAM suppression but also induce an innate antitumor response. Particularly, hyaluronan (HA) is one example of a native extracellular matrix composite found in human tissue, and its accumulation and turnover have been used to modulate inflammatory responses of macrophages [164, 165]. The increase of HA deposition into sites of tissue injury and during inflammatory diseases inspired the incorporation of HA in nanomaterials for immunomodulation in regenerative medicine [166]. Recently, a type of biomimetic and photo-responsive HA hydrogel nanocomposites has been fabricated with tuneable 3D extracellular matrix (ECM) adhesion sites for dynamic macrophage immunomodulation (Figure 7A). Alkoxylphenacyl-based polycarbonate (APP) was utilized to synthesis photodegradable nanocomposites which later attached Arg-Gly-Asp (RGD) for user-controlled peptide release and conjugation to an HA (50 kDa)-based ECM for real-time integrin activation of macrophages encapsulated in 3D HA-APP nanocomposite hydrogels. It was demonstrated that photo-monitoring 3D ECM-RGD peptide conjugations can activate αvβ3 integrins of macrophages, and periodic αvβ3 integrin activation can enhance anti-inflammatory M2 macrophage polarization. The implications of this temporal control over macrophage immunomodulation exemplify a novel approach to control inflammatory responses that may accelerate endogenous tissue repair responses [167]. To this direction, HA biopolymer was encapsulated on MnO2 nanosheet integrated UCNPs to inhibit tumor recurrence by reprogramming TAMs population during post-PDT [168]. Specifically, the release of HA (40 kDa) upon 808nm laser exposure reprogrammed the TAMs activities from pro-tumor M2 to anti-tumor M1 phenotype. They found that treatment of cells with HA-modified nanoparticles enhanced anti-tumor cytokines (e.g., IL-6 and TNF-α) secretion of macrophages and therefore prevented tumor relapse.
It is also important to note that the macrophages reprogramming toward M1 phenotype and proinflammatory gene expression are regulated by stimulation of NF-κB signaling cascades and mitogen-activated protein kinase (MAPK) [169]. Downstream pathways of mitogen-activated protein kinase (MAPK) and NF-κB can be regulated by ROS which oxidize cysteine residue of protein [170]. Specifically, ROS are generated during lipopolysaccharide (LPS) or interferon-γ (IFN-γ) stimulation by NADPH oxidases in M1 signal transduction. Upon entering the cytoplasm, they oxidize the catalytic cysteine of MAPK phosphatase and trigger activation of MAPK cascades including JNK and p38 MAPK [171]. Therefore, activation of M1 signaling pathways can be achieved by precision nanoparticle-based ROS photogeneration in TAMs. Toward this end, mannose-modified PEGylated poly(lactic-co-glycolic acid) (PLGA) nanoparticles were fabricated with the encapsulation of photosensitizers indocyanine green (ICG) and titanium dioxide (TiO2) with ammonium bicarbonate (NH4HCO3) to deliver ROS into endosome/lysosome or cytoplasm of TAMs upon 808 nm laser (0.7 W/cm2) irradiation (Figure 7B-C). Intriguingly, the intracellular ROS photogeneration could switch M2 macrophages to M1 phenotype which was evidenced by the promptly increasing in the expression of M1 markers iNOS and interleukin-12 (IL-12) and the reduction in the levels of M2 markers CD206 and interleukin-10 (IL-10), in a nanoparticle concentration-dependent manner [172].
Light-responsive nanomaterials for macrophage reprograming. (A) Photoresponsive HA hydrogel nanocomposite with photocontrolled 3D ECM adhesion sites for dynamic macrophage immunomodulation (Adapted with permission from [167], copyright 2018 Wiley-VCH). (B) Schematic illustrating the composition/structure of MAN-PLGA nanoparticles and (C) their mechanism of action for ROS photogeneration in endosome/lysosome or cytoplasm to skew M2 macrophages to M1 phenotype inhibiting tumor growth ( B and C were adapted with permission from [172], copyright 2018 American Chemical Society).
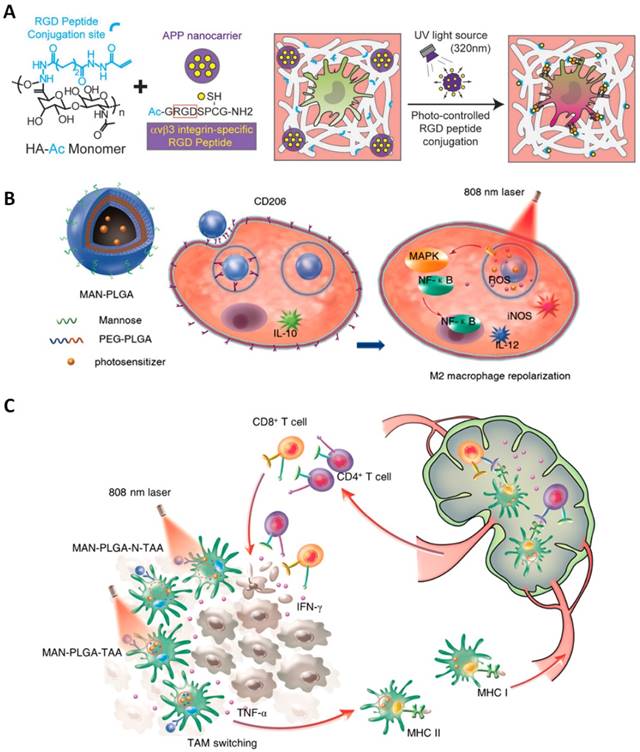
While polarization of macrophage to M1 phenotypes promote fighting against infection and activating anti-inflammatory, polarization to pro-healing M2 phenotypes can stimulate tissue generation and anti-inflammatory reactions. Usually, the polarization of macrophage regulates multiple cellular functions including mechanical, physical cues, transcriptional signal, and chemokines. Therefore, monitoring a balanced macrophage polarization in the living system is crucial in the treatment of various pathologies such as atherosclerosis, obesity, tumor, and asthma [173]. So far, several biomaterials have been used for the regulation of macrophages based on multiples chemical groups [174], mechanical properties, and nanotopography [175]. Intriguingly, it has been reported that calcium ion level is associated with the polarization of macrophages. One recent study using photosensitive NPs for regulating the intracellular Ca2+ concentrations illustrated its possibility of remote-control macrophage polarization. Specifically, UCNPs were used as nanocarriers of calcium supply or calcium chelation compounds. Under NIR irradiation, photolabile linkers was disrupted and therefore enabled release of small molecules for time-regulated control of intracellular calcium levels. The results have also revealed that elevation or depletion of Ca2+ levels could induce polarization of macrophages to M1 or M2 types, respectively [176].
Promisingly, light-mediated modulation of photosensitive NPs can be used for further understanding of cellular functions and processes regarding the correlation with the polarization of macrophage. Furthermore, remote control of phenotypes would allow dynamic regulation of various immune functions in vivo to treat many immune-mediated pathologies.
3.3.3. Optoimmunoengineering
The explosion of genetically encoded light-responsive models has led to the integration of optogenetic and immuno-engineering. By conferring optogenetic tools in immune cells, spatiotemporal precision modulations of DCs maturation, lymphocyte trafficking, inflammasome activities have been achieved in certain extent through interdisciplinary efforts from nanomaterial chemists, immunologists, biologists, and engineers [177]. The advancement of this direction has witnessed the light-controlled migration (phototaxis) of a photoactivatable C-X-C chemokine receptor type 4 (PA-CXCR4) expressing CTLs [178], the light-induced therapeutic T cells expressing chimeric antigen receptor (CAR-T) for minimized “on-target, off-tumor” cytotoxicity [179], and the photosensitive domain to activate Ca2+ influx, GTPases as well as receptor tyrosine kinases [180-182].
Plasma membrane Ca2+ channels and Ca2+ influx are particularly crucial in different steps of the cell-cycle progression and proliferation of immune cells [183]. On the surface of most immune cells, Ca2+ release-activated Ca2+ channels (CRAC channels) present as a natural ion channel which are activated after Ca2+ depletion in the endoplasmic reticulum involving the engagement of immunoreceptors by antigens [184]. He et al. developed an opto-CRAC system mimicking a conformational switch in stromal interaction molecule (STIM1) which could stimulate Ca2+ influx and consequently trigger immune cells response following light illumination [185]. NIR-to-blue emitting UCNPs were utilized with streptavidin-conjugation to enable specific targeting toward cell expressing engineered ORAI channels with extracellular StrepTag. The system was then introduced into therapeutic dendric cells (DCs) in a mouse model of melanoma. NIR light subsequently enabled activation of Opto-CRAC channel to promote DC maturation and antigen presentation which in turn stimulated T cell priming. This approach has paved the way for the high spatiotemporal precision control over engineered T cells and DCs to minimize toxicity as well as off-target cross-reaction during future adoptive immunotherapy.
These studies have illustrated that the coadministration of immune activators, activation of ion channel and monitoring immune suppressive blockers magnificently increase photoimmunotherapy efficiency. Nanoparticles that are capable of utilizing NIR light to provide specific tumor ablation also show its excellence in enhancing tumor antigen release better than chemotherapies. Therefore, nanoparticle-based photoimmunotherapy will lay a promising platform for a highly effective tumor treatment without recurrence, achieving comprehensive cancer immunotherapy.
3.4. Precision cancer phototherapy
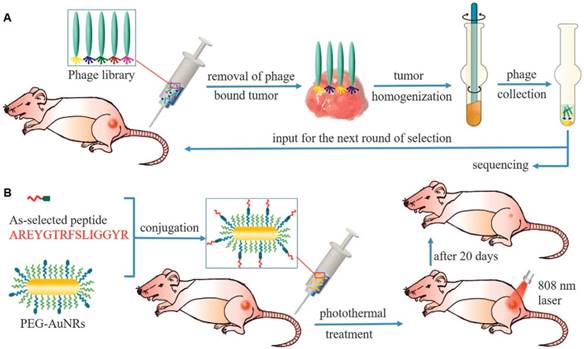
Cancer is currently still one of the most paramount threat in human life [186]. Current advances in photo-responsive nanotechnology for cancer treatment observed highly desire for nanoplatforms with excellent cancer target accuracy for minimizing off-target toxicity [187, 188]. In this context, targeting patient-specific tumors for customized treatment is an advanced goal of precise medicine [189, 190]. This unconventional research area focuses on the personalized tumor distinctions for the same type of cancers, aiming at providing patient-specific drug formulations and treatment solutions. Rationally, each type of cancer cell differs from patient to patient, and tumor receptors for conventional targeting methods are inconsistent in expressing level among patients [191]. Recently, an approach to explore personalized-tumor receptors (named phage display technique) was presented, allowing the selection of peptides that are capable of recognizing an unknown type of tumors (Figure 8). Selection of patient-specific cancer targeting peptides was processed through in vivo phage display. Specifically, a phage library was injected into tumor-bearing mice and allowed to circulate for 24 h. Subsequently, tumor cells with preferentially bound phages were removed out and homogenized. The obtained phage after purification was amplified and used for the next round of selection. After five rounds, the highest tumor affinity peptide was discovered and its excellent accumulation at the tumor site was verified in vivo. Moreover, the selected peptide was conjugated on gold nanorods to enhance targeted-delivery of nanomedicine to the tumor and improve photothermal cancer-killing efficiency. Promisingly, this phage display-based precision medicine would be expanded to other cancer therapeutics such as photodynamic and chemotherapy [192].
Beside specific delivery of nanomaterial to tumor areas, a further step in optimizing precision cancer phototherapy is specifically targeting subcellular compartments of the tumor cell. Of note, many therapeutics are mainly effective in specific organelles [193]. Recent advances in this direction have shown significant progress regarding highly accurate targeting and manipulating subcellular areas such as plasma membrane, cytoplasm, mitochondria, and nucleus.
3.4.1. Plasma membrane targeted phototherapy
The cellular membrane is a fundamental component which is not only responsible for structure function but also involves in the incorporation of signalling, receptors, function proteins, interactions between cells and the environments [194]. Cancer cells membrane normally express specific receptors that are beneficial for the targeting of nanoparticles for precise cancer therapy. Other than that, alteration of lipid composition or membrane fluidity has also approved to significantly affect cancer cell survival [195, 196]. For example, near infrared photoimmunotherapy (NIR-PIT) is a new method of cancer therapy which relies on the activation of photosensitizers conjugated on tumor cell membrane upon NIR irradiation. When antibody photosensitizers specifically bind to antigens on cell membrane and are exposed to NIR laser, selective phototoxicity will be induced. Extensive studies on NIR-PIT have revealed obvious morphological changes in the cell membrane structure during the post-irradiation period. One recent study provided new insight that tiny plasma membrane perforations, which allows ion passing, would stimulate the permeability increase, plasma membrane rupture, and necrotic, immune cell death during NIR-PIT. In contrast with the other claims for ROS which is responsible for the lipid peroxidation and membrane damage, this study on cell structure around early time point during NIR-PIT demonstrated that irreversible minute damage was induced before significant morphological changes apparent [197].
Schematic description of phage display-based breast cancer precision medicine. (A) Selection of patient-specific breast cancer-targeting peptides through in vivo phage display. (B) Coupling of the as-selected peptide with anticancer nanomedicines (AuNRs) to enhance their accumulation inside tumors to achieve highly efficient cancer treatment by photothermal therapy (Adapted with permission from [192], copyright 2017 Nature Publishing Group)
As one essential component of the cell surface, membrane ion channels, which are responsible for the balance of physiological and pathophysiological processes in almost all types of cells including cancer. Reasonably, precise regulation of ion channels would possibly foster manipulation of various cellular activities in high spatiotemporal resolution. This regulation has been approved to trigger the apoptosis process in cancer cells as illustrated in several studies using different external stimuli [198]. Recently, Xing et al. have performed membrane-localized optogenetic monitoring by covalently conjugated UCNPs and engineered Channelrhodopsin-2 [199]. Upon NIR irradiation, the incorporated ion channel induced Ca2+ influx which subsequently enhanced the expression of Caspase-3, one executioner enzyme in apoptosis. Alternatively, ion channel residing on cancer cell membrane have been regulated by photothermal nanoparticles promoting apoptosis cell death. Pu et al. designed organic photothermal nanoagonists (SPN1-C) consisting of semiconducting polymer nanoparticles (SPNs) and agonists, Capsaicin (Cap), for photothermally activation of transient receptor potential cation channel subfamily V member 1 (TRPV1). Under NIR laser irradiation, SPN1-C repeatedly released Cap to multiply activate the TRPV1 channel, causing the influx of ions in mitochondria and in turn specifically induce cancer cell apoptosis which minimizes inflammation and is preferable cancer treatment method in compare with necrotic cell death [200].
In fact, the tumor microenvironments are very complicated that do not allow the homogeneous distribution of therapeutic agents and limit the delivery of anticancer drugs to all the cells residing within tumor areas, and therefore, constraining complete cancer therapy. To overcome this issue, extracellular vesicles packaged with synthetic receptors lipid conjugates (SR-lipids) were produced as cooperative membrane-targeting nanomaterials (referred as fusogenic liposomes, FLs). Via EV-mediated intercellular lipid transfer, SRs were delivered to other tumor cells membrane resulting autonomously spreading throughout tumor tissues (Figure 9A). Therefore, the substantial tumor membrane selective incorporation of SR-lipid could enhance specific and homogeneous accumulation of photosensitizers. In this case, Ce6 photosensitizer was conjugated with streptavidin (SA) then selectively linked with the biotin-FLs to study the membrane photodamage using a 660 nm laser. ROS release from Ce6 upon light irradiation rapidly induced necrosis-like cell death of Ce6 membrane bounded cells while free Ce6-SRs in the medium did not significantly introduce phototoxicity. Collectively, this type of nanoparticles embodied the powerful ability to improve precise tumor therapy [74].
3.4.2. Cytoplasm targeted phototherapy
One critical problem of light-responsive nanotechnology for cancer treatment is the speed of cytoplasmic therapeutic delivery. In fact, external stimuli such as light irradiation have been applied to spatiotemporally disassemble nanoparticles, thereby generating significant drug release inducing anticancer activities [201-203]. However, this process is normally time-consuming and notably affected by the limited lifespan of endocytic compartment trafficking and digestion by lysosomal enzymes [204]. To achieve the biological effects of macromolecules, internalization to the cytosol is crucial in many cases. Photochemical internalization (PCI) is recently used as a promising technology to overcome the membrane barrier of the endocytic vesicles [205]. This technology is mainly based on the use of photosensitizers located in the endocytic vesicles that under light irradiation induces lysosomal membrane disruption and release macromolecules from the compartmentalization [206]. So far, various nanostructures have been constructed relying on this mechanism. Polyion complex vesicles (PICsomes), for example, have been made from a pair of oppositely charged poly(ethylene glycol) (PEG)-based block aniomer and homocationmer. By encapsulating photosensitizers into PICsomes, the PIC membrane have experienced photochemical damage under photoirradiation, resulting in translocation of the photosensitizers and PICsomes themselves to the cytoplasm [207]. Similarly, photothermal Cypate and HSP90 inhibitor 7-allylamino-17-demethoxygeldanamycin (17AAG) were integrated into micelles offering a mutual synergy of photothermal therapy and molecularly targeted therapy. Intriguingly, the release of ROS caused by Cypate under light irradiation has enabled lysosomal disruption and effective cytoplasmic translocation of 17AAG. The translocated inhibitor molecules specifically bind with HSP90, further block stress-induced HSP90 and thereby boosting both early and late apoptosis of Cypate for effective tumor ablation [208].
Other than that, various light activatable nanoparticles have also been explored to promptly release therapeutic payloads [209, 210]. The ability of laser-induced drug release of porphyrin-phospholipid (PoP) liposomes, for example, could be remarkably enhanced by incorporation of small amount of an unsaturated phospholipid, such as dioleoylphosphatidylcholine (DOPC). This allowed a minimum usage of PoP (0.1-0.3 mol%) to promote rapid NIR release of Doxorubicin. The high release rate is attributed to the oxidation of DOPC by singlet oxygen as approved by the control experiment in which oxygen scavenger or antioxidant addition was presented showing light-triggered release inhibition. This study illustrated a strategy of co-incorporation of unsaturated phospholipid with photosensitizers in bilayer nanoparticles for elevating light-trigger cytoplasmic release of therapeutics [211]. Essentially, several limitations regarding complicated design, continuous stimulation due to reversible responses properties are ongoing issues for maximizing cytoplasmic translocation of anticancer compounds. Recently, the drug-encapsulated polymeric nanoparticles of the selenium-inserted copolymer (I/D-Se-NPs) were fabricated providing highly effective synergistic thermo-chemotherapy (Figure 9B). Indocyanine green (ICG) was loaded into the copolymer which triggered ROS induction upon light exposure. The produced ROS played a simultaneous role in inducing selenium oxidation for continuous drug release with irreversible disassembly of nanostructures and mediating lysosomal disruption for augmenting cytoplasmic delivery efficiency. According to the study, this approximately 64 nm polymer nanoparticles rapidly dissociated in several minutes and thus quickly promoted cytoplasmic drug translocation which was then preferably accumulated to the nucleus in only 5 minutes [212].
Subcellular targeted nanoplatforms for precise tumor phototherapy. (A) Schematic representation of cooperative tumor cell membrane targeting nanosystem (Adapted with permission from [74], copyright 2017 Nature Publishing Group). (B) Schematic illustration of selenium-inserted polymeric nanoparticles (I/D-Se-NPs) with immediate drug release and highly efficient cytoplasmic translocation properties under NIR light exposure for synergistic thermo-chemotherapy (Adapted with permission from [212], copyright 2017 American Chemical Society). (C) Fabrication of PF127/me-IR825 NPs and their applications in mitochondrial imaging, cancer/normal cell differentiation, and mitochondria-targeted PTT (Adapted with permission from [221], copyright 2018 American Chemical Society). (D) the pH-responsive and light triggerable nuclear delivery of 10-hydroxycamptothecine using PPR-NPs (Adapted with permission from [237], copyright 2018 America Chemical Society).
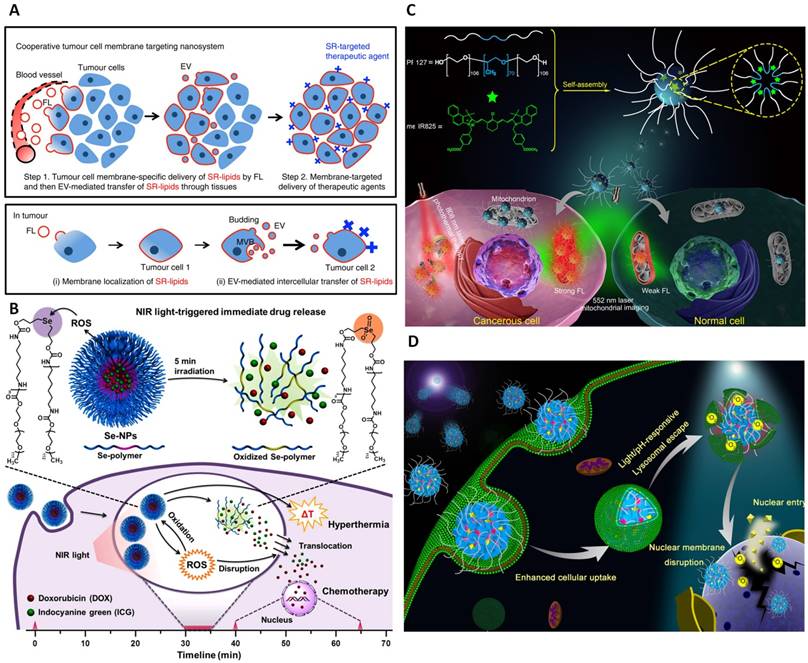
3.4.3. Mitochondria targeted phototherapy
Mitochondria are important organelles responsible for energy production which control multiple cellular processes including epigenetic, differentiation, cellular apoptosis and cell adaptation for microenvironment challenges [213, 214]. Therefore, mitochondrial malfunction is closely related to various diseases including cancer and mitochondrial specific cancer targeting exhibit promising direction for cancer diagnosis and enhancing tumor therapy [215]. Toward this end, multiple approaches have been proposed recently including targeting mitochondrial membrane, mitochondrial ATP, mitochondrial ROS level and mtDNA. These methods showed its superity in precise cancer treatment and overcoming multidrug resistance [216-219]. Importantly, triphenylphosphonium (TPP) is popularly used for introducing the mitochondrial targeting function of nanoparticles. This high lipophilicity moiety was integrated in poly-(ethylene glycol) (PEG)-modified polydopamine nanoparticles (PDA-PEG) which were then loaded with Dox for improving potential of overcoming drug resistance in a long time anticancer therapy [215]. These small targeting molecules also functionalized the coumarin-based fluorescent iron oxide nanoparticles for uplifted hyperthermia and better cytotoxicity in Hella cells in comparison with the results from nanoparticles without mitochondria targeting unit. In agreement with the in vitro studies, 740 nm laser irradiation induced tumor suppression in tumor xenograft mouse models through mitochondria-targeted hyperthermia nanoparticles [220]. Recently, Guang et al. reported the fabrication of theranostic nanoparticles capable of mitochondria targeting which was useful in imaging cancer cells for early-stage diagnostic and mitochondria-specific photothermal therapy (Figure 9C). NIR heptamethine cyanine dye me-IR 825 was encapsulated into the micelle-forming copolymer Pluronic F127 (PF127) to produce nanoparticles with dual channel activatable fluorescent emissions. The emitted 610 nm fluorescence was used for in vitro imaging of cancer mitochondria, distinguishing tumor and normal cell and accurate early stage detection of cancer. Meanwhile, the 845 nm fluorescence was applied for in vivo NIR imaging with high contrast. Furthermore, these nanoparticles exhibited impressive photothermal tumor ablation as proved both in vitro and in vivo. This high biocompatibility and biodegradability nanoparticles guarantee excellent biosafety for mitochondria-targeted cancer theranostics [221]. Besides, mitochondria has similarly shown its highly susceptible to ROS [222]. In a recent study, IR 808 small molecules were anchored on manganese oxide nanoparticles (IR808@MnO NPs) for effective phototherapy of cancer. Particularly, IR808 acted as a tumor cell targeting unit which could relocate nanoparticles in the mitochondria, where MnO reacted with H2O2 to continuously produce oxygen (O2). Under 808 nm laser exposure, the produced O2 was turned into toxic 1O2 and hyperthermia was promoted by IR808 molecules. The highly effective synergistic combination therapy from this nanoplatforms are expected to broaden the application of cancer precise therapy [223].
3.4.4. Nucleus targeted phototherapy
The nucleus is a largest and stiffest organelle in most cells which contain important genetic materials and control various cell activities including cell migration, cancer metastasis [224]. It has been shown that governing nuclear deformability or altering nuclear structure could further mediate cellular functions [225, 226]. For instance, nuclear localization signal (NLS, CGGGPKKKRKVGG) peptide was conjugated for targeting the AuNPs to the nucleus, hence enhancing nuclear membrane stiffness and improving the inhibition effect on cell migration and invasion [227].
Notably, some therapeutic agents such as Doxorubicin could not be efficiently activated unless entering the nucleus [228]. This issue places another important subcellular target, nucleus, in consideration. Many nanomaterials were designed to effectively deliver drugs or photoactive agents into tumor cells, escaping from endo/lysosome to the cytosol using either intracellular or extracellular stimuli [229-231]. Unfortunately, most of these nanoagents cannot pass the nuclear pore complexes with a small size of around 9 to 12 nm [227]. Several studies to overcome this barrier have fabricated positive charge nanoparticles to increase the electrostatic interaction with the nucleus [232, 233]. Nuclear localization sequences have been designed in targeting the nuclear translocation property of importin, which then integrated with nanocarriers for effective nuclear localization [234-236]. Recently, self-assembled nanoparticles (PEG-POSS-RB-NPs denoted as PPR-NPs) were introduced by conjugating multiamine-containing polyhedral oligomeric silsesquioxane (POSS) molecule with a hydrophilic polyethylene glycol (PEG) chain and several hydrophobic photosensitizers, rose bengal (RB) molecules (Figure 9D). Benefiting from the positive charge given by the amine groups on the surface, these NPs could easily enter the cell and be entrapped by lysosomes. After irradiating with light, 1O2 generation caused significant lysosomal disruption and thereby released PPR-NPs into cytosol. The exposing POSS molecules with polyamine property substantially directed the nanoparticles toward nuclear membranes, which furthermore, enhanced production of 1O2 disintegrated the nuclear membrane, encouraging the nuclear entry of the PPR-NPs. It was evidenced that this method could not only deliver chemotherapeutics (10-hydroxycamptothecine and docetaxel) but also nanomaterials themselves without conventional chemical modification [237].
At present, many breakthroughs have been discovered in the innovation of light-activated nanomaterials for the early diagnosis and treatment of cancers. Tumor-specific targeting that relies on the tumor-associated antigens, and proteins has achieved considerable advances. More importantly, subcellular compartment targeting has gained intensive attention. These methods offer efficient and highly precise theragnostic results with a lower dosage of therapeutic agents. With intensive investigations on small molecules, macro biomolecules, cellular behaviors and processes as well as the innovation of optical and nanomaterial techniques, improvements in this area are believed to proliferate in near future for treatment of not only local but also metastasis tumors.
3.5. Other cell functions facilitating
The profound advantages of light-responsive nanoparticles have also been extensively applied in studies of various other biomedical concerns such as coronary artery [238], cardiovascular [239], diabetes [240] and vision restoration [241]. Particularly, gold nanoparticles-decorated titania nanowire arrays were developed recently as an artificial photoreceptor for the restoration of visual response in blind mice [241]. Such innovation sheds light on the promising direction for solving a considerable challenge of the past decade regarding complex feature in retinal degenerative diseases. Moreover, light-based nanosystems could be exploited in targeting other subcellular targets which required a high level of accuracy such as RNA, DNA, and cytoskeleton. Thereby, these high spatiotemporal resolution light-regulated systems could regulate various essential cellular processes including gene scissoring, transgene expression, cytoskeletal regulations, as well as cells migrations, and interactions.
Light-activatable nanoparticles for manipulation of cell functions. (A) Schematic illustration of chiral CdTe-based specific DNA cleavage under circular polarized light irradiation (Adapted with permission from [242], copyright 2018 Nature Publishing Group). (B) Illustration of dendronized semiconducting polymers (DSP) -mediated gene delivery and remote photothermal activation of gene expression under NIR laser irradiation at 808 nm (Adapted with permission from [243], copyright 2017 Wiley-VCH). (C) Illustration of nanoscale optomechanical actuators for controlling mechanotransduction in living cells and time-lapse images of NIH/3T3 cell migration in response to light stimulation. The white dashed circles represent the region of NIR illumination (Adapted with permission from [244], copyright 2015 Nature Publishing Group). (D) Schematic for proposed mechanism of AuNRs and plasmonic photothermal therapy in inhibiting cancer collective migration (Adapted with permission from [245], copyright 2018 America Chemical Society).
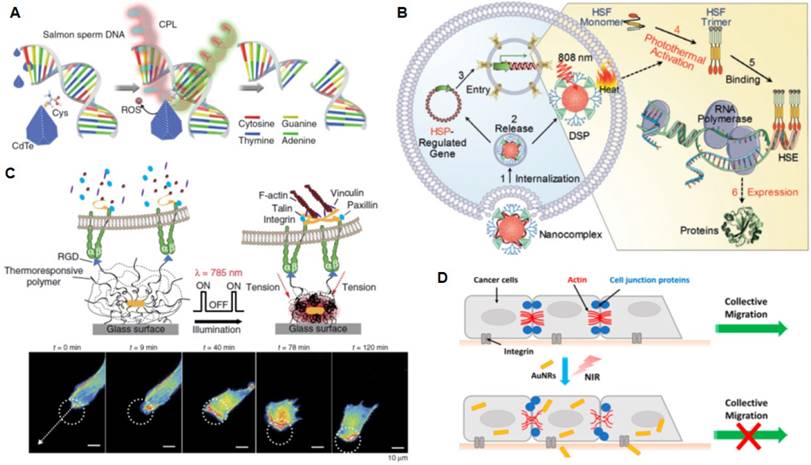
Gene therapy has become a promising therapeutic approach in the treatment of inherited genetic diseases. Precise modulation of gene activities at a designated time and location is currently a direction for minimizing off-target side effects [246]. Several methods have been proposed toward this track including gas molecules, radio waves, but light is still one of the most ideal inducers [247,248]. Innovatively, semiconducting cadmium telluride (CdTe) nanoparticles were used as an artificial biomimetic DNA endonuclease that could facilitate the sequence-specific cutting of nucleic acids (Figure 10A). The nanoparticles with truncated tetrahedral shape were coated with chiral cysteine producing nanomaterials that can specifically recognize the restriction site (GATATC) in a DNA sequence of certain length (above 90 base pairs). When being illuminated with polarized light, the attached NPs acted as photosensitizers and produced the hydroxyl radicals that cut the phosphodiester bonds which linked the sugar moieties between GAT and ATC units in the backbone of the nucleic acid strand. An array of experiments was conducted both in vitro and in vivo to confirm the specificity of the approach, in terms of composition and cutting location. This innovative nanomaterials carried out themselves the cutting ability and represented a new pathway for the synthesis of inorganic enzymes applicable in other biological systems and shed light on the development of gene analysis and therapies [242].
Remote activation of gene expression could also be manipulated by photo-activatable nanoparticles [249]. Toward this goal, dendronized semiconducting polymers (DSP) constituted of NIR photothermal transducer, the gene vectors, and water solubility enhancers were fabricated to regulate heat sock promoter (HSP70) for gene expression [243]. Gene of interest cloned downstream of HSP70 was incorporated in a plasmid that recognized cues generated from photothermal signals. Through electrostatic interaction of DSP with HSP70-regulated gene, nanocomplexes were induced. Upon intracellular up-taking, these nanocomplexes released gene into the nucleus and maintained the DSP in the cytoplasm. After 808 nm laser illumination, heat that was generated from DSP induced heat shock factors and triggered a chain of processes to stimulate the transcription and transgene expression (Figure 10B). This approach provided an insight of innovatively control gene expression at predetermined time and location with minimal invasiveness, thus held great promise in nanomedicine application of monitored therapeutic transgene dosing.
Recently, the clustered regularly interspaced short palindromic repeat (in short as CRISPR) associated proteins 9 (CRISPR/ Cas9) has emerged as a most powerful gene-editing platform. Using RNAs for specific-site recognition, this system can precisely disrupt, insert, or replace a DNA sequence at a specific locus in the genome which offers effective genomic alteration method and active genetic drives for the elimination of diseases [250]. While the detailed mechanism of CRISPR/ Cas9 is still under intensive investigation, biomedical engineers have focused on optimizing delivery systems for these gene-editing elements as well as precisely controlling its activities under dose, time and space for critical applications [251]. To this direction, light has contributed a high spatiotemporal resolution modulation of Cas9 system which enables noninvasive precise genomic modifications. The light activated system consisting of cryptochrome 2 (CRY2) and calcium and integrin binding protein 1 (CIB1) which can be heterodimerized under exposure of blue light and separated under dark condition was used as a robust, time-dependent regulation of a targeted gene. This designed was proposed by Polstein et al. and Nihongaki et al. with the similar fusing of dCas9 with CIB1 and a transcriptional activator domain (VP64 or p65) with the CRY2 domain [252]. Moreover, the activity of Cas9 was also precisely monitored by engineering a photoactivatable split Cas9. For example, positive magnet (pMag) and negative magnet (nMag) was added in N- and C-terminal fragments of Cas9 offering blue light reversible modulation of nuclease activities and precise genomic modifications. In contrast, the psCas9 system developed by Zhou et al incorporated photo-dissociable dimeric fluorescent protein pdDronpa1 into the REC2 and PI domains of Cas9. Upon irradiation with 500nm light, gene editing functions of Cas9 was activated which was inhibited in the dark due to the dimerization of pdDronpa1 domains [253]. More recently, far-red light activated CRISPR-dCas9 effector (FACE) system based on dCas9 and the bacterial phytochrome BphS was developed [254]. This robust device has been demonstrated to mediate targeted epigenetic modulation and shown its significant in promoting differentiation of induced pluripotent stem cells into functional neurons by up-regulating a single neural transcription factor, NEUROG2. Such optogenetic toolkit expanded the way for precise spatiotemporal control of cellular behaviours and boosted the clinical progress of precision optogenetics-based therapy. Nevertheless, efficient delivery of the CRISPR-Cas9 system into a specific position was still one of the critical issues that limited its application in vivo. For overcoming this hurdle, light-responsive nanocomplexes hold great potential with respect to safety, large packaging capacity, multifunctional ability. For instance, Cas9-sgPlk-1 plasmids (CP) was condensed on TAT peptide-modified Au nanoparticles to produce LACP for tumor therapy. After entering tumor cells, CP can be released into cytosol by laser-triggered thermo-effects of AuNPs. Under TAT guidance, CPs are able to enter nuclei, knock out targeted Plk-1 gene and inhibit the tumor growth [255]. In combination with interventional therapeutic techniques, this design could be extended for a novel carrier for delivering various Cas9-sgRNAs and treating a broad spectrum of diseases by gene editing.
The cytoskeleton is an important cell structural protein. Changing in the structure of this target is correlated with affecting cell migration and metastasis. Therefore, cytoskeleton has become a promising focus on innovative cancer therapy. This goal sparked several studies on inhibiting cancer metastasis focusing on the migration of a single cell using light-responsive nanoparticles. Moustafa et al. illustrated that integrin targeted AuNPs, which produced mild heat after applying NIR irradiation, decreased the cell migration speed, therefore providing the potential application for monitoring cancer metastasis [256]. In another work, gold nanorods were coated with a thermo-responsive polymer that rapidly shrank upon NIR illumination, thereby applying mechanical force to manipulate receptor mechanics with unprecedented spatiotemporal resolution [244]. In this system, gold nanorods performed as photothermal transducers which converted NIR light to heat and physically drove force to control focal adhesion (Figure 10C). Series of experiments revealed that the particles heating was transient and confined to the core and the mechanical energy was efficiently transmitted with negligible increase in surface temperature which minimized the thermal effect on cells. The mechanical force exerted was around 13-50 pN that suitable for stimulating cell migration and T cell activation. This nontoxic optomechanical actuator nanoparticles that delivered precise physical inputs to the biological system would probably broaden the avenue for the studies of mechanotransduction, precluding genetic modification for extended in vivo applications.
Cytoskeleton filaments interaction could also be targeted for more efficient cancer metastasis treatment. This is based on the fact that a group of cancer cells normally migrates together, regarded as collective cancer metastasis that uses the active cell-cell junction from the interaction of cytoskeleton filaments [257]. The multicellular integration process, which requires cytoskeleton connection of neighbour cells, is highly dynamic and regulated by signal transduction through protein phosphorylation. Recently, this route was inhibited by integrating AuNRs in collective cancer cells and applying plasmonic photothermal therapy [245] (Figure 10D). Supposedly, the interaction between integrin-targeted AuNRs and cells triggered the phosphorylation alteration of signals associated with cytoskeleton filaments, which in turn drove the morphological changes and constrain cancer collective migration.
4. Conclusion and perspective
Light-triggered nanotechnology has so far approved its importance in precision medicine. While other extracellular or intracellular stimulus-responsive theranostic systems have still encountered challenges of ineffectiveness and side effects, light-receptive nanomaterials have offered minimal-invasive and high spatiotemporal resolution strategies in accurate biomedical applications. To date, multiple photo-controlled drug delivery, photodynamic and photothermal nanoplatforms have been fabricated employing a wide spectrum of light from UV to NIR region. Of note, recent development in this area has been witnessing the emergence of NIR-activatable nanocomplexes which overcomes short-wavelength light disadvantages of autofluorescence, phototoxicity, and limited penetration depth. Such advent has perceived tremendous contributions from robust inorganic nanomaterials such as AuNPs, UCNPs, highly biodegradable, biocompatible organic nanocomplexes as well as biological-based nanosystems. Table 1 summarizes recent representative light-responsive nanoparticles that have been employed for precise regulation of cellular events and disease treatments. Among those, neuromodulation has gained enormous benefits from remote manipulation of neuronal responses and wireless control of animal behaviours; various immunological cells have also been stimulated for elevation of photoimmunotherapy and optoimmunoengineering; stem cells have been regulated by light, mediating its extracellular conditions and delivery of guided small molecules which is significantly promising for future tissue repairing and regenerative medicine. In addition, cancer therapy observed incredible improvement with the exploitations of light-manipulate nanotherapy providing photothermal, photodynamic, photoimmunological therapies in a highly precise manner of targeting personalized tumor cells and specific tumour cell compartments. Besides, the application of light-responsive nanoparticles could be broadened for other cell functions such as migration, interactions and transcription of genes which brighten the perspective of comprehensive remote-controlled medicine. It is irrefutable that the innovation of nanoparticles fabrication techniques in integration with light and the revolution of optogenetic engineering have greatly met the ever-demanding precise control of biological events. Nevertheless, clinical applications of these platforms still need to overcome several difficulties before benefiting public healthcare system.
First, most activities exploiting light-based nanoparticles are hindered by the intrinsic disadvantages of light in a living organism which are nonspecific reactivity and limited penetration depth, especially those utilizing UV-Vis wavelength for stimulations. Indeed, UV light emits high-energy photons that are absorbed by multiple biological molecules, resulting in a remarkable diminishing of penetration depth and increasing the possibility of photochemical reactions at the non-targeting tissue or subcellular areas. Therefore, conventional utilization of UV light activatable inorganic particles like QDs or other organic molecules leads to noticeable side effects that interfere with their efficiency, specificity, and biosafety. In fact, great efforts have been made to tackle this issue such as applying continuous UV lasers instead of broad-band light or exploiting two-photon excitation method for activation of photosensitive moieties. However, significance of these alternations is still limited as it is not applicable for all moieties and nanoplatforms. Elsewhere, this difficulty can be moderately alleviated by either modifying chemical structure to enhance light sensitivity or fabricate ultra-small nanoparticles for reducing background signals [258]. In addition, lowering tissue scattering by employing NIR light is another option because of its biological-friendly properties giving minimal photodamage to the tissue and remarkable penetration depth. One noteworthy attempt in that direction is the preparation of red-shifted photoactivated chromophores and materials such as tricyanofuran, DANP, BODIPY, red-shifted azobenzene with impressive biomedical applications [259]. Although the great promising in principle, the low quantum efficiency of some modified chromophores might hinder its biomedical applications. Moreover, nanoparticles with the performance rely on NIR light such as AuNPs and UCNPs should be paid more attention to photothermal efficiency as well as quantum yields. Normally, the photo-conversion efficiency of UCNPs is suboptimal for satisfying practical applications. In order to enhance upconversion efficiency, optimization of the lattice structure of UCNPs received intense interests such as changing the structure to be excited under 808 nm instead of 980 nm wavelength that hitting the water absorbance peak. Moreover, quantum efficiency and excitation wavelength engineering of UCNPs can be improved by integrating with alternative sensitizers, host matrices, or dye antennas [102, 260, 261]. AuNPs can be synthesized in different sizes and shapes for varying their plasmon resonance peaks [262]. Alternatively, recent advancement in organic polymer-based NPs is promising for establishing more efficient photothermal conversion for higher biocompatibility and more effective treatments. It is also reported that nanomaterials such as graphene, CNTs, AuNPs, PdNPs or CuS, MoS2 are all able to provide NIR-to-heat conversion [263]. Of note, red-shifted AuNPs induced by designed aggregation have shown enhanced NIR-triggered photothermal efficiency [264]. Furthermore, structure and surface modification of NPs such as producing CuS nanoflowers or coupling of Cu2-x Se to CNTs can increase the photothermal efficiency and lower the incident power density. In fact, low power densities produce less damage to healthy cells compared to laser beam alone. Recently, extensive studies have used NIR irradiation and two-photon excitation process. Two-photon NIR systems, for example, offer a remarkable 3D spatial resolution with much deeper tissue penetration depth, inhibition of premature cargos release and less oxidative cytotoxicity. Nevertheless, the introduction of two-photon excitation leads to another issue regarding the complicated synthesis of chromophores. Compounds such as [7-(diethylamino) coumarin-4-yl]methyl esters with strong electron donating and accepting groups in a highly conjugated system are highly applicable in two-photon platforms with its significant two-photon cross-section [265]. Promisingly, these strategies would not only enhance the photoactivation capacity of photo-assisted nanoplatforms but also minimize the detrimental side effects induced by short wavelength light, high power density light. Importantly, they add in further opportunities in orthogonal regulation of nanomedicine.
Second, the biosafety and long-term toxicity are other major concerns of nanotechnology to be clinically available. The behaviors of nanoparticles in biological systems significantly affect their future biomedical uses which depend on multiple factors including size, shape, stability, and chemical functions. The accumulation and attachment of nanoparticles on the cell surface, tissues or protein, enzymes could also potentially restrict the natural cellular processes such as regulatory and cell-cell communication functions. Additionally, the retained nanoparticles in a biological system may possibly cause an increase in oxidation stress and expression of pro-inflammatory gene [266]. Many nanomaterials, especially inorganic NPs with generally higher stability, are non-biodegradable which retain a long time inside the body and possibly cause durable side effects. To alleviate this issue, various modification approaches are supposed to increase the nanoparticles biocompatibility such as polymer or silica coating. The polymer coating on UCNPs, for example, can increase its gene transfection efficiency and reduce cytotoxicity. The development of polymeric and bio-mimetic NPs recently would offer exciting opportunities for minimizing toxicity and nonspecific uptake [267, 268]. These polymers such as polypyrroles possess remarkable drug encapsulation, facile modification ability with enhanced control over biodistribution and degradation kinetics, high stability and biocompatibility, with neglected long-term cytotoxicity [269]. Other than that, organic dyes such as ICG could be easily cleared in the biological system and hence highly beneficial for biomedical applications. These photoactivated agents have been approved by the FDA for clinical uses [270]. Notably, the light-controlled treatments and regulations require predetermined location and site of irradiation while in many situations, the exact position of disease cells are unknown such as in metastasis cancer or therapeutic T cells cases. Thus, it is highly desirable for innovation of the multifunctional NPs which provide precise imaging and targeting of the specific tissues. Additionally, other challenges that administered NPs would face in the body are potential immune response, serum protein binding or cellular interaction with normal tissue, hepatobiliary and renal excretion [267]. In order to get FDA approval for human use, these limitations are needed to be considered carefully unless otherwise NPs may encounter prohibitive regulatory hurdles. The shape and size of the NPs should be strictly put into attention. For successful administer of NPs, their hydrodynamic diameters should be larger than 5-6 nm to avoid removal by renal clearance systems , and meanwhile, they should be smaller than 200 nm to avoid clearance by the liver and the spleen [63]. It has been reported that AuNPs with varied size and shape could significantly affect its biodistribution and properties in the biological environment. For example, studies on in vitro responses of different size and shape of siRNA conjugated AuNPs demonstrated that 50 nm sphere and 40 nm stars showed much higher uptake efficiency compared to 13 nm spheres. At early incubation time, all three forms were localized in the endosomes. However, after 24 h incubation, 50 nm spheres and 40 nm stars were not detected in either endosomes or lysosomes while 13 nm spheres stayed in endosomes [271]. Therefore, intensive studies on the morphological characteristics of NPs might offer outstanding regulation effect towards cellular properties [272]. Furthermore, surface chemistry and charge also play pivotal roles in biodistribution and pharmacokinetic properties as well as cytotoxicity of NPs. Generally, a near neutral charge of NPs is preferable, and modification of the NPs surface can alter the properties of the whole system. For that, polyethylene glycol (PEG) to its surface (PEGylation) are popularly used for offering neutral charge NPs and allowing long circulation time, to prevent opsonization and phagocytosis by the cells of the reticuloendothelial system (e.g., kupffer cells, phagocytes) [273]. For specific targeting and eliminating side effects, targeting moieties (e.g. antibodies, aptamers, small molecules, etc.) have been widely exploited. Particularly, monoclonal IgG antibodies (mAbs) with two epitope binding sites, high selectivity and binding affinity have been widely used for protein recognition and targeting [274]. Meanwhile, aptamers, which are folded single strand oligonucleotides, 25-100 nucleotides in length (8-25 kDa), can selectively bind to molecular targets through high throughput screening methods [274, 275]. Additionally, small molecules including folic acid, transferrin, and the RGD peptide sequence have been extensively used for surface modification of many NPs. Especially, RGD is a component of the extracellular matrix protein fibronectin which plays an essential role in promoting cell adhesion and regulating cell growth, proliferation, and migration. It is well known that a cyclic peptide containing the RGD sequence also has high affinity to integrins [276]. Therefore, modification of NPs with cyclic RGD would offer integrin targeting properties and enabling cellular regulation through integrin intervention.
Although significant development of light-activatable nanosystems has been made toward physio-chemical, pharmacokinetic, biodistribution, there are many challenges appeared in pre-clinical studies. In particular, variation in the cell lines, microenvironment factors, lack of systematic pharmacokinetics data are limitations to progression in this field.
Thirdly, one fundamental roadblock that restrains the application of photo-activated nanosystems is the engineering strategy. Specifically, facile synthesis of light-activated nanoparticles with sufficient post-synthesis stability is of great concern. Particularly, synthesis of NIR light-responsive UCNPs can be followed by many different protocols such as hydrothermal-assisted template method, sol-gel processes, polymer graphing, electrospinning, self-assembly, or one step ligand exchange. By adjusting the synthesis time, temperature, composition, the properties of UCNPs can be tuned regarding its lifetime, size, shape as well as luminescent properties. Especially, the one-step ligand exchange method could eliminate the alternation of interface charge equilibration and hence increase the UCNPs stability in aqueous solutions. Likewise, various attempts in the synthesis of QDs have been witnessed recently. Among those, some significant improvement such as aqueous phase synthesis, large-scale synthesis, green and facile synthesis are promising for the QDs applications in industrial perspective. The highly clinical applicable polymer nanoparticles can be fabricated by various methods including layer-by-layer self-assembling, radical polymerization technique, or reversible addition-fragmentation chain transfer polymerization. Currently, conventional methods to install light sensitivities such as ChRs or optogenetic tools into the cell of interest have suffered from multiple rounds of trial and error. Therefore, intensively investigation of targeted optogene delivery systems should be considered to streamline this process in routine clinical use. Moreover, establishing synthesis procedure and scale-up NPs with controllable reproducibility for commercial applications are still challenging, especially for organic and biological-based NPs regarding the quantity and cost. In fact, approaches have been reported to boost the clinical translations namely lentiviral infection technique for specific cell/tissue optogenetic toolboxes engineering [277] or top-down lithographic method for replicate synthesis of polymeric NPs [278]. In spite of that, further research in this area is still highly demanding for successful bench-to-bedside transition and real-world translations.
Representative light-responsive nanoparticles for diverse biomedical functions.
| Type of nanomaterials | Size | Wavelength | Functions | Photo-activate mechanisms | Ref. | |
|---|---|---|---|---|---|---|
| Inorganic NPs | AuNR@SiO2 | (L/W):3.4 ± 1.4 | 780 nm | Stimulation of primary auditory neurons | Photothermal | [109] |
| AuNRs@catHDL | Not given | 780 nm | Ca2+ influx by TRPV1 activation in neuronal cells | Photothermal | [114] | |
| AuNRs@NH2-PEG | L, W: 18.5 nm, 71.3 nm | 785 nm | Inhibiting the electrical activity of neuron by activation of TREK-1 thermosensitive potassium channel | Photothermal | [115] | |
| UCNPs | D: 90nm | 980 nm | Deep brain manipulation of neuronal activities. | ChR2 or Arch photoactivation | [103] | |
| UCNP@Ce6-R837 | D: 80nm | 980 nm | Eliminating tumor, long-term immune memory functions. | Photodynamic, photoimmunotherapy | [161] | |
| UCNPs | D: 45 nm | 980 nm | Regulating the function of non-excitable cells, including T lymphocytes, macrophages and dendritic cells | Photoactivation of engineered Opto-CRAC channels | [185] | |
| UCNT@mSiO2-βCD-RGD | D: 38±4 nm | 980 nm | Inducing differentiation of hMSCs into hypertrophic chondrocytes, chondrocytes or osteoblasts by regulating Ca2+ intracellular concentration. | Photocleavage induced KGN release | [142] | |
| PEGlated- CuNWs | D: 46 nm; L: 40 µm | 808 nm | Directing cancer ablation and activate effector T cells | Photothermal | [279] | |
| CuS-CpG | D: 85nm | 900 nm | Tumor cell death, tumor antigens release, reduce tumor growth | Photothermal and immunotherapy | [156] | |
| CdTe | 4.5 ± 0.3 nm | 405 nm | Selective DNA cutting between T and A bases of the restriction site GATATC | Photoactivation | [242] | |
| Organic NPs | SPNbc | D: 25 nm | 808 nm | Neuronal control by activation of TRPV1 | Photothermal | [117] |
| PLGA-ICG-R837 | D: 100 nm | 808 nm | Primary tumor ablation, checkpoint-blockades, metastasis inhibition | Photothermal-immunotherapy | [157] | |
| NCP@pyrolipid | D: 55.3±0.2 nm | 670 nm | Tumor cells killing, strong tumor-specific immune response. | Photodynamic-immunotherapy | [160] | |
| GITR-PLGA | D: 142.6 -183.0 nm | 808 nm | Tumor apoptosis/necrosis, Treg cell suppression, immune response | Photothermal-photodynamic-photoimmunotherapy | [81] | |
| MAN-PLGA | D: 78 nm | 808 nm | Eradicating tumors, reprogramming TAMs to an antitumor M1 phenotype, preventing recurrence. | Photodynamic- photoimmunotherapy | [172] | |
| Biomimetic NPs | Fusogenic liposomes (FLs) | D: 126.5 nm | 660 nm | Cooperating with EVs to distribute synthetic receptors-lipid throughout tumor tissues and improve the photosensitive agents' therapeutic responses | Photodynamic | [74] |
| Extracellular vesicles- ChR2 (EVs-ChR2) | D: 100-1000 nm | 480 nm | Transferring engineered ion channel to recipient cells and stimulate its Ca2+ signaling pathways | Photoactivation of ChR2 | [71] | |
| Exosomes | D:186.8 nm (90.8%) | 460 nm | Intracellular delivery of proteins for protein-based therapeutics | Photoactivation of CRY2-CIB1 | [75] | |
Note: D: diameter; L: length; W: width
Finally, further understanding of cellular functions and enhancement of instrumentations are of critical importance. In particular, studies on elucidating cellular activities mechanism would provide new insights in manipulating different cell functions with minimized invasiveness and low dependence of artificial cellular engineering. Take ion regulation for example, even the evolutions of ChR-based optogenetic tools are still on the journey, other native ion stimulation pathways such as STIM-ORAI or membrane proteins ion mediation systems as well as inherent ion channels still require more in-depth and comprehensive studies. Regarding delivery of functional proteins, ion channels, or genes into targeted cells, recent advancement in cellular-derived nanoparticles such as exosomes and extracellular vesicles hold noticeable future perspective toward excluding genetic expression. In addition, designing suitable medical devices such as optical fibers, endoscopy holds great importance for local light activation of nanosystems with real-time accurate tracking and monitoring biological processes. In line with this, commercialized instruments with significant reproducibility and robustness for light-mediated nanotheranostics are highly demanding. Incorporation of advanced spectrometers, microscopes, and stimulators in this multidisciplinary area requires cross-continental cooperation which is also critical for its real-world applications.
In summary, the development of light-responsive nanoplatforms so far holds bright future for the interrogations of highly spatiotemporal stimulation of cellular events with precise disease theranostics from single cell level up to entire animals. It is expected that the advancement of current innovative nanotechnology would provide more biocompatible nanosystems with excellent specific targeting, activating and enriching a new generation of therapeutic platforms. In combination with varying optical modals options from optimal wavelength choice of light to the novelty of instrumentations, light-controlled nanomedicine is approaching further steps toward the ultimate goal of serving clinical practices.
Acknowledgements
This work was partially supported by Tier 1 RG 5/18 (S) and RG110/16 (S), NTU-JSPS JRP grant (M4082175.110), SSIJRI (203-A018003), Merlion program (M4082162.110), Merlion program (M408110000), MOE 2017-T2-2-110 awarded in the Nanyang Technological University, Singapore.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Son J, Yi G, Yoo J, Park C, Koo H, Choi HS. Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv Drug Deliv Rev. 2019:133-47
2. Genchi GG, Marino A, Grillone A, Pezzini I, Ciofani G. Remote control of cellular functions: The role of smart nanomaterials in the medicine of the future. Adv Healthc Mater. 2017;6:1700002
3. Blum AP, Kammeyer JK, Rush AM, Callmann CE, Hahn ME, Gianneschi NC. Stimuli-responsive nanomaterials for biomedical applications. J Am Chem Soc. 2015;137:2140-54
4. Pierini F, Nakielski P, Urbanek O, Pawłowska S, Lanzi M, De Sio L. et al. Polymer-based nanomaterials for photothermal therapy: From light-responsive to multifunctional nanoplatforms for synergistically combined technologies. Biomacromolecules. 2018;19:4147-67
5. Zhao Q, Wang Y, Yan Y, Huang J. Smart nanocarrier: Self-assembly of bacteria-like vesicles with photoswitchable cilia. ACS Nano. 2014;8:11341-9
6. Barnett SC, Perry BAL, Dalrymple-Alford JC, Parr-Brownlie LC. Optogenetic stimulation: Understanding memory and treating deficits. Hippocampus. 2018;28:457-70
7. Hoffmann MD, Bubeck F, Eils R, Niopek D. Controlling cells with light and LOV. Adv Biosyst. 2018;2:1800098
8. Yun SH, Kwok SJJ. Light in diagnosis, therapy and surgery. Nat Biomed Eng. 2017;1:0008
9. Shao N, Qi Y, Lu H, He D, Li B, Huang Y. Photostability highly improved nanoparticles based on IR-780 and negative charged copolymer for enhanced photothermal therapy. ACS Biomater Sci Eng. 2019;5:795-804
10. Li S, Liu R, Jiang X, Qiu Y, Song X, Huang G. et al. Near-infrared light-triggered sulfur dioxide gas therapy of cancer. ACS Nano. 2019;13:2103-13
11. Wang C, Dai C, Hu Z, Li H, Yu L, Lin H. et al. Photonic cancer nanomedicine using the near infrared-II biowindow enabled by biocompatible titanium nitride nanoplatforms. Nanoscale Horiz. 2019;4:415-25
12. Yang Y, Shao Q, Deng R, Wang C, Teng X, Cheng K. et al. In vitro and in vivo uncaging and bioluminescence imaging by using photocaged upconversion nanoparticles. Angew Chem Int Ed. 2012;51:3125-9
13. Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380-6
14. Dayem AA, Choi HY, Yang GM, Kim K, Saha SK, Kim JH. et al. The potential of nanoparticles in stem cell differentiation and further therapeutic applications. Biotechnol J. 2016;11:1550-60
15. Roberts JE. Light and immunomodulation. Ann N Y Acad Sci. 2000;917:435-45
16. Zhang P, Hu C, Ran W, Meng J, Yin Q, Li Y. Recent progress in light-triggered nanotheranostics for cancer treatment. Theranostics. 2016;6:948-68
17. Gupta A, Avci P, Sadasivam M, Chandran R, Parizotto N, Vecchio D. et al. Shining light on nanotechnology to help repair and regeneration. Biotechnol Adv. 2013;31:607-31
18. Journal of the American Chemical SocietyTrends ImmunolTong R, Kohane DS. Shedding light on nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:638-62
19. Issa MC, Manela-Azulay M. Photodynamic therapy: a review of the literature and image documentation. An Bras Dermatol. 2010;85:501-11
20. Huang Z, Xu H, Meyers AD, Musani AI, Wang L, Tagg R. et al. Photodynamic therapy for treatment of solid tumors-potential and technical challenges. Technol Cancer Res Treat. 2008;7:309-20
21. Jaque D, Martínez Maestro L, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL. et al. Nanoparticles for photothermal therapies. Nanoscale. 2014;6:9494-530
22. Ellis-Davies GCR. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619-28
23. Bao Z, Liu X, Liu Y, Liu H, Zhao K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. AJPS. 2016;11:349-64
24. Huang J, Guo M, Ke H, Zong C, Ren B, Liu G. et al. Rational design and synthesis of ɤFe2O3@Au magnetic gold nanoflowers for efficient cancer theranostics. Adv Mater. 2015;27:5049-56
25. Chen CW, Lee PH, Chan YC, Hsiao M, Chen CH, Wu PC. et al. Plasmon-induced hyperthermia: hybrid upconversion NaYF4:Yb/Er and gold nanomaterials for oral cancer photothermal therapy. J Mater Chem B. 2015;3:8293-302
26. Jalani G, Tam V, Vetrone F, Cerruti M. Seeing, Targeting and delivering with upconverting nanoparticles. J Am Chem Soc. 2018;140:10923-31
27. Lin X, Chen X, Zhang W, Sun T, Fang P, Liao Q. et al. Core-shell-shell upconversion nanoparticles with enhanced emission for wireless optogenetic inhibition. Nano Lett. 2018;18:948-56
28. Pliss A, Ohulchanskyy TY, Chen G, Damasco J, Bass CE, Prasad PN. Subcellular optogenetics enacted by targeted nanotransformers of near-infrared light. ACS Photonics. 2017;4:806-14
29. Shen J, Chen G, Vu A-M, Fan W, Bilsel OS, Chang C-C. et al. Engineering the upconversion nanoparticle excitation wavelength: Cascade sensitization of tri-doped upconversion colloidal nanoparticles at 800 nm. Adv Opt Mater. 2013;1:644-50
30. Chen G, Damasco J, Qiu H, Shao W, Ohulchanskyy TY, Valiev RR. et al. Energy-cascaded upconversion in an organic dye-sensitized core/shell fluoride nanocrystal. Nano Lett. 2015;15:7400-7
31. Liu TM, Conde J, Lipiński T, Bednarkiewicz A, Huang CC. Smart NIR linear and nonlinear optical nanomaterials for cancer theranostics: Prospects in photomedicine. Prog Mater Sci. 2017;88:89-135
32. Hu F, Zhang Y, Chen G, Li C, Wang Q. Double-walled Au nanocage/SiO2 nanorattles: integrating SERS imaging, drug delivery and photothermal therapy. Small. 2015;11:985-93
33. Zhang Y, Li J, Jiang H, Zhao C, Wang X. Rapid tumor bioimaging and photothermal treatment based on GSH-capped red fluorescent gold nanoclusters. RSC Adv. 2016;6:63331-7
34. Saverot S, Geng X, Leng W, Vikesland PJ, Grove TZ, Bickford LR. Facile, tunable, and SERS-enhanced HEPES gold nanostars. RSC Adv. 2016;6:29669-73
35. Lee JW, Jung H, Cho HH, Lee JH, Nam Y. Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials. 2018;153:59-69
36. Gai S, Yang G, Yang P, He F, Lin J, Jin D. et al. Recent advances in functional nanomaterials for light-triggered cancer therapy. Nano today. 2018;19:146-87
37. Goo S, Choi YJ, Lee Y, Lee S, Chung HW. Selective Effects of Curcumin on CdSe/ZnS Quantum-dot-induced Phototoxicity Using UVA Irradiation in Normal Human Lymphocytes and Leukemia Cells. Toxicol Res. 2013;29:35-42
38. Lee SH, Lee HB, Kim Y, Jeong JR, Lee MH, Kang K. Neurite Guidance on Laser-Scribed Reduced Graphene Oxide. Nano Lett. 2018;18:7421-7
39. Chen R, Zhang J, Wang Y, Chen X, Zapien JA, Lee CS. Graphitic carbon nitride nanosheet@metal-organic framework core-shell nanoparticles for photo-chemo combination therapy. Nanoscale. 2015;7:17299-305
40. Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373-87
41. Yu J, Yin W, Peng T, Chang YN, Zu Y, Li J. et al. Biodistribution, excretion, and toxicity of polyethyleneimine modified NaYF4:Yb,Er upconversion nanoparticles in mice via different administration routes. Nanoscale. 2017;9:4497-507
42. Kuche K, Maheshwari R, Tambe V, Mak KK, Jogi H, Raval N. et al. Carbon nanotubes (CNTs) based advanced dermal therapeutics: current trends and future potential. Nanoscale. 2018;10:8911-37
43. Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri SM, Mirshekari H. et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457-501
44. Shi Y, Shi B, Dass AVE, Lu Y, Sayyadi N, Kautto L. et al. Stable Upconversion Nanohybrid Particles for Specific Prostate Cancer Cell Immunodetection. Sci Rep. 2016;6:37533
45. Ng KK, Zheng G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem Rev. 2015;115:11012-42
46. Song X, Zhang R, Liang C, Chen Q, Gong H, Liu Z. Nano-assemblies of J-aggregates based on a NIR dye as a multifunctional drug carrier for combination cancer therapy. Biomaterials. 2015;57:84-92
47. Bhattacharya P, Du D, Lin Y. Bioinspired nanoscale materials for biomedical and energy applications. J R Soc Interface. 2014;11:20131067
48. Brown PK, Qureshi AT, Moll AN, Hayes DJ, Monroe WT. Silver nanoscale antisense drug delivery system for photoactivated gene silencing. ACS Nano. 2013;7:2948-59
49. Karthik S, Puvvada N, Kumar BN, Rajput S, Pathak A, Mandal M. et al. Photoresponsive coumarin-tethered multifunctional magnetic nanoparticles for release of anticancer drug. ACS Appl Mater Interfaces. 2013;5:5232-8
50. Tan X, Li BB, Lu X, Jia F, Santori C, Menon P. et al. Light-triggered, self-immolative nucleic Acid-drug nanostructures. J Am Chem Soc. 2015;137:6112-5
51. Tong R, Hemmati HD, Langer R, Kohane DS. Photoswitchable Nanoparticles for Triggered Tissue Penetration and Drug Delivery. J Am Chem Soc. 2012;134:8848-55
52. Li W, Chen Z, Zhou L, Li Z, Ren J, Qu X. Noninvasive and Reversible Cell Adhesion and Detachment via Single-Wavelength Near-Infrared Laser Mediated Photoisomerization. J Am Chem Soc. 2015;137:8199-205
53. Guo X, Wang L, Wang S, Li Y, Zhang F, Song B. et al. Synthesis of new chlorin derivatives containing maleimide functional group and their photodynamic activity evaluation. Bioorg Med Chem Lett. 2015;25:4078-81
54. Shivran N, Tyagi M, Mula S, Gupta P, Saha B, Patro BS. et al. Synthesis and photodynamic activity of some glucose-conjugated BODIPY dyes. Eur J Med Chem. 2016;122:352-65
55. Sakr MH, Halabi NM, Kalash LN, Al-Ghadban SI, Rammah MK, El Sabban ME. et al. Synthesis and in vitro cytotoxicity evaluation of ruthenium polypyridyl-sensitized paramagnetic titania nanoparticles for photodynamic therapy. RSC Adv. 2016;6:47520-9
56. Lim CK, Shin J, Kwon IC, Jeong SY, Kim S. Iodinated photosensitizing chitosan: Self-assembly into tumor-homing nanoparticles with enhanced singlet oxygen generation. Bioconjug Chem. 2012;23:1022-8
57. Muhanna N, Jin CS, Huynh E, Chan H, Qiu Y, Jiang W. et al. Phototheranostic porphyrin nanoparticles enable visualization and targeted treatment of head and neck cancer in clinically relevant models. Theranostics. 2015;5:1428-43
58. Cui L, Lin Q, Jin CS, Jiang W, Huang H, Ding L. et al. A PEGylation-free biomimetic porphyrin nanoplatform for personalized cancer theranostics. ACS Nano. 2015;9:4484-95
59. Sheng Z, Hu D, Zheng M, Zhao P, Liu H, Gao D. et al. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy. ACS Nano. 2014;8:12310-22
60. Zhou J, Lu Z, Zhu X, Wang X, Liao Y, Ma Z. et al. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials. 2013;34:9584-92
61. Yang K, Xu H, Cheng L, Sun C, Wang J, Liu Z. In Vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv Mat. 2012;24:5586-92
62. Ryu JH, Messersmith PB, Lee H. Polydopamine surface chemistry: A decade of discovery. ACS Appl Mater Interfaces. 2018;10:7523-40
63. Stylianopoulos T, Jain RK. Design considerations for nanotherapeutics in oncology. Nanomedicine. 2015;11:1893-907
64. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. PNAS. 2011;108:10980-5
65. Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO. et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61-8
66. Pawlowski CL, Li W, Sun M, Ravichandran K, Hickman D, Kos C. et al. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials. 2017;128:94-108
67. Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P. et al. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403-9
68. Brillault L, Jutras PV, Dashti N, Thuenemann EC, Morgan G, Lomonossoff GP. et al. Engineering recombinant virus-like nanoparticles from plants for cellular delivery. ACS Nano. 2017;11:3476-84
69. Gao W, Hu C-MJ, Fang RH, Luk BT, Su J, Zhang L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25:3549-53
70. Li X, Zhao H, Ji Y, Yin C, Li J, Yang Z. et al. Lysosome-assisted mitochondrial targeting nanoprobe based on dye-modified upconversion nanophosphors for ratiometric imaging of mitochondrial hydrogen sulfide. ACS Appl Mater Interfaces. 2018;10:39544-56
71. Lyu L, Hu M, Fu A, Xing B. Extracellular vesicle directed exogenous ion channel transport for precise manipulation of biological events. Bioconjug Chem. 2018;29:2715-22
72. Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE. et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319
73. Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A. et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6:1324730
74. Kim H, Lee J, Oh C, Park JH. Cooperative tumour cell membrane targeted phototherapy. Nat Commun. 2017;8:15880
75. Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H. et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277
76. Gujrati V, Prakash J, Malekzadeh-Najafabadi J, Stiel A, Klemm U, Mettenleiter G. et al. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat Commun. 2019;10:1114
77. Yoo J-W, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10:521
78. Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2016;2:16075
79. Chen H, Gu Z, An H, Chen C, Chen J, Cui R. et al. Precise nanomedicine for intelligent therapy of cancer. Sci China Chem. 2018;61:1503-52
80. Gong H, Cheng L, Xiang J, Xu H, Feng L, Shi X. et al. Near-infrared absorbing polymeric nanoparticles as a versatile drug carrier for cancer combination therapy. Adv Funct Mater. 2013;23:6059-67
81. Ou W, Jiang L, Thapa RK, Soe ZC, Poudel K, Chang JH. et al. Combination of NIR therapy and regulatory T cell modulation using layer-by-layer hybrid nanoparticles for effective cancer photoimmunotherapy. Theranostics. 2018;8:4574-90
82. Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9:1074
83. Liu F, He X, Lei Z, Liu L, Zhang J, You H. et al. Facile preparation of doxorubicin-loaded upconversion@polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy. Adv Healthc Mater. 2015;4:559-68
84. Yan W, Xu L, Xu C, Ma W, Kuang H, Wang L. et al. Self-assembly of chiral nanoparticle pyramids with strong R/S optical activity. J Am Chem Soc. 2012;134:15114-21
85. Sun M, Hao T, Li X, Qu A, Xu L, Hao C. et al. Direct observation of selective autophagy induction in cells and tissues by self-assembled chiral nanodevice. Nat Commun. 2018;9:4494
86. Gao F, Sun M, Ma W, Wu X, Liu L, Kuang H. et al. A singlet oxygen generating agent by chirality-dependent plasmonic shell-satellite nanoassembly. Adv Mat. 2017;29:1606864
87. Ma W, Sun M, Fu P, Li S, Xu L, Kuang H. et al. A chiral-nanoassemblies-enabled strategy for simultaneously profiling surface glycoprotein and microRNA in living cells. Adv Mat. 2017;29:1703410
88. Hassler C, Boretius T, Stieglitz T. Polymers for neural implants. J Polym Sci B Polym Phys. 2011;49:18-33
89. Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275-309
90. Histed MH, Ni AM, Maunsell JH. Insights into cortical mechanisms of behavior from microstimulation experiments. Prog Neurobiol. 2013;103:115-30
91. MacDougall M, Nummela SU, Coop S, Disney A, Mitchell JF, Miller CT. Optogenetic manipulation of neural circuits in awake marmosets. J Neurophysiol. 2016;116:1286-94
92. Zhang K, Cui B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 2015;33:92-100
93. Duebel J, Marazova K, Sahel J-A. Optogenetics. Curr Opin Ophthalmol. 2015;26:226-32
94. Batabyal S, Cervenka G, Birch D, Kim YT, Mohanty S. Broadband activation by white-opsin lowers intensity threshold for cellular stimulation. Sci Rep. 2015;5:17857
95. Wietek J, Beltramo R, Scanziani M, Hegemann P, Oertner TG, Wiegert JS. An improved chloride-conducting channelrhodopsin for light-induced inhibition of neuronal activity in vivo. Sci Rep. 2015;5:14807
96. Huber D, Petreanu L, Ghitani N, Ranade S, Hromádka T, Mainen Z. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61-4
97. Royer S, Zemelman BV, Barbic M, Losonczy A, Buzsaki G, Magee JC. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur J Neurosci. 2010;31:2279-91
98. Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li Y. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211-6
99. Shah S, Liu J-J, Pasquale N, Lai J, McGowan H, Pang ZP. et al. Hybrid upconversion nanomaterials for optogenetic neuronal control. Nanoscale. 2015;7:16571-7
100. Hososhima S, Yuasa H, Ishizuka T, Hoque MR, Yamashita T, Yamanaka A. et al. Near-infrared (NIR) up-conversion optogenetics. Sci Rep. 2015;5:16533
101. Lin X, Wang Y, Chen X, Yang R, Wang Z, Feng J. et al. Multiplexed optogenetic stimulation of neurons with spectrum-selective upconversion nanoparticles. Adv Healthc Mater. 2017;6:1700446
102. Wu X, Zhang Y, Takle K, Bilsel O, Li Z, Lee H. et al. Dye-sensitized core/active shell upconversion nanoparticles for optogenetics and bioimaging applications. ACS Nano. 2016;10:1060-6
103. Chen S, Weitemier AZ, Zeng X, He L, Wang X, Tao Y. et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science. 2018;359:679-84
104. Eom K, Kim J, Choi JM, Kang T, Chang JW, Byun KM. et al. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small. 2014;10:3853-7
105. Shapiro MG, Homma K, Villarreal S, Richter C-P, Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun. 2012;3:736
106. Paviolo C, Haycock JW, Cadusch PJ, McArthur SL, Stoddart PR. Laser exposure of gold nanorods can induce intracellular calcium transients. J Biophotonics. 2014;7:761-5
107. Thompson AC, Stoddart PR, Jansen ED. Optical stimulation of neurons. Curr Mol Imaging. 2014;3:162-77
108. Carvalho-de-Souza JL, Treger JS, Dang B, Kent SB, Pepperberg DR, Bezanilla F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron. 2015;86:207-17
109. Paviolo C, McArthur SL, Stoddart PR. Gold nanorod-assisted optical stimulation of neuronal cells. J Vis Exp. 2015;98:e52566
110. Yong J, Needham K, Brown WG, Nayagam BA, McArthur SL, Yu A. et al. Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. Adv Healthc Mater. 2014;3:1862-8
111. Roper DK, Ahn W, Hoepfner M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J Phys Chem C. 2007;111:3636-41
112. Yong J, Needham K, Brown WGA, Nayagam BA, McArthur SL, Yu A. et al. Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. Adv Healthc Mater. 2014;3:1862-8
113. Paviolo C, Haycock JW, Yong J, Yu A, Stoddart PR, McArthur SL. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol Bioeng. 2013;110:2277-91
114. Nakatsuji H, Numata T, Morone N, Kaneko S, Mori Y, Imahori H. et al. Thermosensitive ion channel activation in single neuronal cells by using surface-engineered plasmonic nanoparticles. Angew Chem Int Ed. 2015;54:11725-9
115. Yoo S, Hong S, Choi Y, Park J-H, Nam Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano. 2014;8:8040-9
116. Lavoie-Cardinal F, Salesse C, Bergeron É, Meunier M, De Koninck P. Gold nanoparticle-assisted all optical localized stimulation and monitoring of Ca2+ signaling in neurons. Sci Rep. 2016;6:20619
117. Lyu Y, Xie C, Chechetka SA, Miyako E, Pu K. Semiconducting polymer nanobioconjugates for targeted photothermal activation of neurons. J Am Chem Soc. 2016;138:9049-52
118. Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci. 2016;19:554
119. Fukuda N, Matsuda T, Nagai T. Optical control of the Ca2+ concentration in a live specimen with a genetically encoded Ca2+-releasing molecular tool. ACS Chem Biol. 2014;9:1197-203
120. Feldbauer K, Schlegel J, Weissbecker J, Sauer F, Wood PG, Bamberg E. et al. Optochemokine tandem for light-control of intracellular Ca2+. PloS One. 2016;11:e0165344
121. Rich TC, Webb KJ, Leavesley SJ. Can we decipher the information content contained within cyclic nucleotide signals? J Gen Physiol. 2014;143:17-27
122. Rost BR, Schneider-Warme F, Schmitz D, Hegemann P. Optogenetic tools for subcellular applications in neuroscience. Neuron. 2017;96:572-603
123. Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos Trans R Soc Lond B Biol Sci. 2010;365:155-63
124. Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7:195-207
125. Dong Y, A S, Rodrigues M, Li X, Kwon SH, Kosaric N. et al. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv Funct Mater. 2017;27:1606619
126. Yates CC, Nuschke A, Rodrigues M, Whaley D, Dechant JJ, Taylor DP. et al. Improved transplanted stem cell survival in a polymer gel supplemented with tenascin C accelerates healing and reduces scarring of murine skin wounds. Cell Transplant. 2017;26:103-13
127. Yamazoe T, Shiraki N, Toyoda M, Kiyokawa N, Okita H, Miyagawa Y. et al. A synthetic nanofibrillar matrix promotes in vitro hepatic differentiation of embryonic stem cells and induced pluripotent stem cells. J Cell Sci. 2013;126:5391-9
128. Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Mol Ther. 2007;15:467-80
129. Lyssiotis CA, Lairson LL, Boitano AE, Wurdak H, Zhu S, Schultz PG. Chemical control of stem cell fate and developmental potential. Angew Chem Int Ed. 2011;50:200-42
130. Andersen MØ, Le DQS, Chen M, Nygaard JV, Kassem M, Bünger C. et al. Spatially controlled delivery of siRNAs to stem cells in implants generated by multi-component additive manufacturing. Adv Funct Mater. 2013;23:5599-607
131. Shah B, Yin PT, Ghoshal S, Lee K-B. Multimodal magnetic core-shell nanoparticles for effective stem-cell differentiation and imaging. Angew Chem Int Ed. 2013;52:6190-5
132. Li J, Lee WY, Wu T, Xu J, Zhang K, Li G. et al. Multifunctional quantum dot nanoparticles for effective differentiation and long-term tracking of human mesenchymal stem cells in vitro and in vivo. Adv Healthc Mater. 2016;5:1049-57
133. Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC. et al. A stem cell-based approach to cartilage repair. Science. 2012;336:717-21
134. Hu Q, Ding B, Yan X, Peng L, Duan J, Yang S. et al. Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine. 2017;13:2189-98
135. Kang ML, Ko JY, Kim JE, Im GI. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35:9984-94
136. Xu J, Li J, Lin S, Wu T, Huang H, Zhang K. et al. Nanocarrier-mediated codelivery of small molecular drugs and siRNA to enhance chondrogenic differentiation and suppress hypertrophy of human mesenchymal stem cells. Adv Funct Mater. 2016;26:2463-72
137. Li J, Lee WY, Wu T, Xu J, Zhang K, Hong Wong DS. et al. Near-infrared light-triggered release of small molecules for controlled differentiation and long-term tracking of stem cells in vivo using upconversion nanoparticles. Biomaterials. 2016;110:1-10
138. Chen R, Wang J, Liu C. Biomaterials act as enhancers of growth factors in bone regeneration. Adv Funct Mater. 2016;26:8810-23
139. Holle AW, Young JL, Van Vliet KJ, Kamm RD, Discher D, Janmey P. et al. Cell-extracellular matrix mechanobiology: forceful tools and emerging needs for basic and translational research. Nano Lett. 2018;18:1-8
140. Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15:326-34
141. Yan Z, Qin H, Ren J, Qu X. Photocontrolled multidirectional differentiation of mesenchymal stem cells on an upconversion substrate. Angew Chem Int Ed. 2018;57:11182-7
142. Kang H, Zhang K, Pan Q, Lin S, Wong DSH, Li J. et al. Remote control of intracellular calcium using upconversion nanotransducers regulates stem cell differentiation in vivo. Adv Funct Mater. 2018;28:1802642
143. Pchelintseva E, Djamgoz MBA. Mesenchymal stem cell differentiation: Control by calcium-activated potassium channels. J Cell Physiol. 2018;233:3755-68
144. Lee MN, Hwang H-S, Oh S-H, Roshanzadeh A, Kim J-W, Song JH. et al. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp Mol Med. 2018;50:142
145. Tiwari JN, Seo YK, Yoon T, Lee WG, Cho WJ, Yousuf M. et al. Accelerated bone regeneration by two-photon photoactivated carbon nitride nanosheets. ACS Nano. 2017;11:742-51
146. Wang Z, Hu M, Ai X, Zhang Z, Xing B. Near-infrared manipulation of membrane ion channels via upconversion optogenetics. Adv Biosyst. 2019;3:1800233
147. Li Z, Song W, Rubinstein M, Liu D. Recent updates in cancer immunotherapy: a comprehensive review and perspective of the 2018 China Cancer Immunotherapy Workshop in Beijing. J Hematol Oncol. 2018;11:142
148. Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L. et al. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from "silent" to immunogenic. Cancer Res. 2007;67:7941-4
149. Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353-63
150. Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P. et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3:e955691
151. Huang H-C, Pigula M, Fang Y, Hasan T. Immobilization of photo-immunoconjugates on nanoparticles leads to enhanced light-activated biological effects. Small. 2018;14:1800236
152. Ogawa M, Tomita Y, Nakamura Y, Lee MJ, Lee S, Tomita S. et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8:10425-36
153. Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25-39
154. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974-82
155. Park K. Combined therapy of imatinib and an anti-CTLA4 immune-checkpoint inhibitor. J Control Release. 2018;281:196
156. Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O. et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 2014;8:5670-81
157. Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193
158. Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mat. 2014;26:8154-62
159. Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR. et al. Chlorin-based nanoscale metal-organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J Am Chem Soc. 2016;138:12502-10
160. He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR. et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7:12499
161. Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X. et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 2017;11:4463-74
162. De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer cell. 2013;23:277-86
163. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13
164. Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41-56
165. Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101
166. Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 2016;10:633-47
167. Wang H, Morales RT, Cui X, Huang J, Qian W, Tong J. et al. A photoresponsive hyaluronan hydrogel nanocomposite for dynamic macrophage immunomodulation. Adv Healthc Mater. 2018;8:e1801234
168. Ai X, Hu M, Wang Z, Lyu L, Zhang W, Li J. et al. Enhanced cellular ablation by attenuating hypoxia status and reprogramming tumor-associated macrophages via NIR light-responsive upconversion nanocrystals. Bioconjug Chem. 2018;29:928-38
169. Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280-8
170. Kamata H, Honda S-i, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649-61
171. Kotsias F, Hoffmann E, Amigorena S, Savina A. Reactive oxygen species production in the phagosome: impact on antigen presentation in dendritic cells. Antioxid Redox Signal. 2013;18:714-29
172. Shi C, Liu T, Guo Z, Zhuang R, Zhang X, Chen X. Reprogramming tumor-associated macrophages by nanoparticle-based reactive oxygen species photogeneration. Nano Lett. 2018;18:7330-42
173. Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520-9
174. Chen Z, Bachhuka A, Han S, Wei F, Lu S, Visalakshan RM. et al. Tuning chemistry and topography of nanoengineered surfaces to manipulate immune response for bone regeneration applications. ACS Nano. 2017;11:4494-506
175. Luu TU, Gott SC, Woo BW, Rao MP, Liu WF. Micro- and Nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl Mater Interfaces. 2015;7:28665-72
176. Kang H, Zhang K, Wong DSH, Han F, Li B, Bian L. Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials. 2018;178:681-96
177. Jeanbart L, Swartz MA. Engineering opportunities in cancer immunotherapy. PNAS. 2015;112:14467-72
178. Schulz O, Hammerschmidt SI, Moschovakis GL, Forster R. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu Rev Immunol. 2016;34:203-42
179. Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077
180. Kyung T, Lee S, Kim JE, Cho T, Park H, Jeong YM. et al. Optogenetic control of endogenous Ca(2+) channels in vivo. Nat Biotechnol. 2015;33:1092-6
181. Kim N, Kim JM, Lee M, Kim CY, Chang KY, Heo WD. Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chem Bio. 2014;21:903-12
182. Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV. Optogenetic protein clustering and signaling activation in mammalian cells. Nat Methods. 2013;10:249
183. Takuwa N, Zhou W, Kumada M, Takuwa Y. Ca2+/calmodulin is involved in growth factor-induced retinoblastoma gene product phosphorylation in human vascular endothelial cells. FEBS lett. 1992;306:173-5
184. Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S. et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435-45
185. He L, Zhang Y, Ma G, Tan P, Li Z, Zang S. et al. Near-infrared photoactivatable control of Ca(2+) signaling and optogenetic immunomodulation. eLife. 2015;4:e10024
186. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30
187. Liu B, Li C, Cheng Z, Hou Z, Huang S, Lin J. Functional nanomaterials for near-infrared-triggered cancer therapy. Biomater Sci. 2016;4:890-909
188. Chitgupi U, Qin Y, Lovell JF. Targeted nanomaterials for phototherapy. Nanotheranostics. 2017;1:38-58
189. Schork NJ. Personalized medicine: Time for one-person trials. Nature. 2015;520:609-11
190. van der Wijst MGP, de Vries DH, Brugge H, Westra HJ, Franke L. An integrative approach for building personalized gene regulatory networks for precision medicine. Genome Med. 2018;10:96
191. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793-5
192. Qu X, Qiu P, Zhu Y, Yang M, Mao C. Guiding nanomaterials to tumors for breast cancer precision medicine: From tumor-targeting small-molecule discovery to targeted nanodrug delivery. NPG Asia Mater. 2017;9:e452
193. Zeng X, Morgenstern R, Nystrom AM. Nanoparticle-directed sub-cellular localization of doxorubicin and the sensitization breast cancer cells by circumventing GST-mediated drug resistance. Biomaterials. 2014;35:1227-39
194. Zalba S, ten Hagen TLM. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat Rev. 2017;52:48-57
195. Garg S, Dhavala S, Krumova K, Kiebish M, Vishnudas V, Gesta S. et al. Membrane fluidity in cancer cell membranes as a therapeutic target: Validation using BPM 31510. Biophys J. 2015;108:246a
196. Tan LTH, Chan KG, Pusparajah P, Lee WL, Chuah LH, Khan TM. et al. Targeting membrane lipid: A potential cancer cure? Front Pharmacol. 2017;8:12
197. Nakajima K, Takakura H, Shimizu Y, Ogawa M. Changes in plasma membrane damage inducing cell death after treatment with near-infrared photoimmunotherapy. Cancer Sci. 2018;109:2889-96
198. Zhang K, Cui B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 2015;33:92-100
199. Ai X, Lyu L, Zhang Y, Tang Y, Mu J, Liu F. et al. Remote regulation of membrane channel activity by site-specific localization of lanthanide-doped upconversion nanocrystals. Angew Chem Int Ed. 2017;56:3031-5
200. Zhen X, Xie C, Jiang Y, Ai X, Xing B, Pu K. Semiconducting photothermal nanoagonist for remote-controlled specific cancer therapy. Nano Lett. 2018;18:1498-505
201. Wang J, Liu Y, Ma Y, Sun C, Tao W, Wang Y. et al. NIR-activated supersensitive drug release using nanoparticles with a flow core. Adv Funct Mater. 2016;26:7516-25
202. Sun X, Wang C, Gao M, Hu A, Liu Z. Drug Delivery: Remotely controlled red blood cell carriers for cancer targeting and near-infrared light-triggered drug release in combined photothermal-chemotherapy. Adv Funct Mater. 2015;25:2480
203. Rwei AY, Wang W, Kohane DS. Photoresponsive nanoparticles for drug delivery. Nano today. 2015;10:451-67
204. Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano today. 2014;9:223-43
205. Berg K, Weyergang A, Prasmickaite L, Bonsted A, Hogset A, Strand MT. et al. Photochemical internalization (PCI): a technology for drug delivery. Methods Mol Biol. 2010;635:133-45
206. Ye S, Rao J, Qiu S, Zhao J, He H, Yan Z. et al. Rational design of conjugated photosensitizers with controllable photoconversion for dually cooperative phototherapy. Adv Mat. 2018;30:1801216
207. Chen H, Xiao L, Anraku Y, Mi P, Liu X, Cabral H. et al. Polyion complex vesicles for photoinduced intracellular delivery of amphiphilic photosensitizer. J Am Chem Soc. 2014;136:157-63
208. Luo H, Wang Q, Deng Y, Yang T, Ke H, Yang H. et al. Mutually synergistic nanoparticles for effective thermo-molecularly targeted therapy. Adv Funct Mater. 2017;27:1702834
209. Shanmugam V, Selvakumar S, Yeh CS. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem Soc Rev. 2014;43:6254-87
210. Meng Z, Wei F, Wang R, Xia M, Chen Z, Wang H. et al. NIR-laser-switched in vivo smart nanocapsules for synergic photothermal and chemotherapy of tumors. Adv Mater. 2016;28:245-53
211. Luo D, Li N, Carter KA, Lin C, Geng J, Shao S. et al. Rapid light-triggered drug release in liposomes containing small amounts of unsaturated and porphyrin-phospholipids. Small. 2016;12:3039-47
212. Wang Y, Deng Y, Luo H, Zhu A, Ke H, Yang H. et al. Light-responsive nanoparticles for highly efficient cytoplasmic delivery of anticancer agents. ACS Nano. 2017;11:12134-44
213. Hou XS, Wang HS, Mugaka BP, Yang GJ, Ding Y. Mitochondria: promising organelle targets for cancer diagnosis and treatment. Biomater Sci. 2018;6:2786-97
214. Gogvadze V. Targeting mitochondria in fighting cancer. Curr Pharm Des. 2011;17:4034-46
215. Li WQ, Wang Z, Hao S, He H, Wan Y, Zhu C. et al. Mitochondria-targeting polydopamine nanoparticles to deliver doxorubicin for overcoming drug resistance. ACS Appl Mater Interfaces. 2017;9:16793-802
216. Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780-8
217. Mkandawire MM, Lakatos M, Springer A, Clemens A, Appelhans D, Krause-Buchholz U. et al. Induction of apoptosis in human cancer cells by targeting mitochondria with gold nanoparticles. Nanoscale. 2015;7:10634-40
218. Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol Res. 2015;100:170-4
219. Wang DF, Rong WT, Lu Y, Hou J, Qi SS, Xiao Q. et al. TPGS2k/PLGA nanoparticles for overcoming multidrug resistance by interfering mitochondria of human alveolar adenocarcinoma cells. ACS Appl Mater Interfaces. 2015;7:3888-901
220. Jung HS, Han J, Lee JH, Lee JH, Choi JM, Kweon HS. et al. Enhanced NIR radiation-triggered hyperthermia by mitochondrial targeting. J Am Chem Soc. 2015;137:3017-23
221. Guang Yu P, Jia H, Zhu YX, Sun W, Cheng X, Wu FG. Cyanine-containing polymeric nanoparticles with imaging/therapy-switchable capability for mitochondria-targeted cancer theranostics. ACS Appl Nano Mater. 2018;1:2885-2897
222. Bae SK, Heo CH, Choi DJ, Sen D, Joe EH, Cho BR. et al. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson's disease gene knockout astrocytes. J Am Chem Soc. 2013;135:9915-23
223. Zhou L, Wu Y, Meng X, Li S, Zhang J, Gong P. et al. Dye-anchored MnO nanoparticles targeting tumor and inducing enhanced phototherapy effect via mitochondria-mediated pathway. Small. 2018;14:e1801008
224. Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol. 2013;23:R1113-21
225. Kirby TJ, Lammerding J. Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol. 2018;20:373-81
226. McGregor AL, Hsia CR, Lammerding J. Squish and squeeze-the nucleus as a physical barrier during migration in confined environments. Curr Opin Cell Biol. 2016;40:32-40
227. Ali MRK, Wu Y, Ghosh D, Do BH, Chen K, Dawson MR. et al. Nuclear membrane-targeted gold nanoparticles inhibit cancer cell migration and invasion. ACS Nano. 2017;11:3716-26
228. Lin X, Li Q, Wang YJ, Ju YW, Chi ZQ, Wang MW. et al. Morphine inhibits doxorubicin-induced reactive oxygen species generation and nuclear factor кB transcriptional activation in neuroblastoma SH-SY5Y cells. Biochem J. 2007;406:215-21
229. Zhang N, Zhao F, Zou Q, Li Y, Ma G, Yan X. Multitriggered tumor-responsive drug delivery vehicles based on protein and polypeptide coassembly for enhanced photodynamic tumor ablation. Small. 2016;12:5936-43
230. Cheng Y, Yumul RC, Pun SH. Virus-inspired polymer for efficient in vitro and in vivo gene delivery. Angew Chem Int Ed. 2016;55:12013-7
231. Karimi M, Sahandi Zangabad P, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR. Smart nanostructures for cargo delivery: Uncaging and activating by light. J Am Chem Soc. 2017;139:4584-610
232. Xu P, Van Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew Chem Int Ed. 2007;46:4999-5002
233. Xiao Y, Shi K, Qu Y, Chu B, Qian Z. Engineering Nanoparticles for Targeted Delivery of Nucleic Acid Therapeutics in Tumor. Mol Ther Methods Clin Dev. 2019;12:1-18
234. Mout R, Ray M, Yesilbag Tonga G, Lee YW, Tay T, Sasaki K. et al. Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein for Efficient Gene Editing. ACS Nano. 2017;11:2452-8
235. Han K, Zhang W-Y, Zhang J, Lei Q, Wang S-B, Liu J-W. et al. Acidity-triggered tumor-targeted chimeric peptide for enhanced intra-nuclear photodynamic therapy. Adv Funct Mater. 2016;26:4351-61
236. Han SS, Li ZY, Zhu JY, Han K, Zeng ZY, Hong W. et al. Dual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small. 2015;11:2543-54
237. Zhu YX, Jia HR, Pan GY, Ulrich NW, Chen Z, Wu FG. Development of a light-controlled nanoplatform for direct nuclear delivery of molecular and nanoscale materials. J Am Chem Soc. 2018;140:4062-70
238. Ambesh P, Campia U, Obiagwu C, Bansal R, Shetty V, Hollander G. et al. Nanomedicine in coronary artery disease. Indian Heart J. 2017;69:244-51
239. Jiang W, Rutherford D, Vuong T, Liu H. Nanomaterials for treating cardiovascular diseases: A review. Bioact Mater. 2017;2:185-98
240. DiSanto RM, Subramanian V, Gu Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:548-64
241. Tang J, Qin N, Chong Y, Diao Y, Yiliguma, Wang Z. et al. Nanowire arrays restore vision in blind mice. Nat Commun. 2018;9:786
242. Sun M, Xu L, Qu A, Zhao P, Hao T, Ma W. et al. Site-selective photoinduced cleavage and profiling of DNA by chiral semiconductor nanoparticles. Nat Chem. 2018;10:821-30
243. Lyu Y, Cui D, Sun H, Miao Y, Duan H, Pu K. Dendronized semiconducting polymer as photothermal nanocarrier for remote activation of gene expression. Angew Chem Int Ed. 2017;56:9155-9
244. Liu Z, Liu Y, Chang Y, Seyf HR, Henry A, Mattheyses AL. et al. Nanoscale optomechanical actuators for controlling mechanotransduction in living cells. Nat Methods. 2015;13:143
245. Wu Y, Ali MRK, Dong B, Han T, Chen K, Chen J. et al. Gold nanorod photothermal therapy alters cell junctions and actin network in inhibiting cancer cell collective migration. ACS Nano. 2018;12:9279-90
246. Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351
247. Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, Friedman JM. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science. 2012;336:604-8
248. Folcher M, Oesterle S, Zwicky K, Thekkottil T, Heymoz J, Hohmann M. et al. Mind-controlled transgene expression by a wireless-powered optogenetic designer cell implant. Nat Commun. 2014;5:5392
249. Nakatsuji H, Kawabata Galbraith K, Kurisu J, Imahori H, Murakami T, Kengaku M. Surface chemistry for cytosolic gene delivery and photothermal transgene expression by gold nanorods. Sci Rep. 2017;7:4694
250. Pineda M, Moghadam F, Ebrahimkhani MR, Kiani S. Engineered CRISPR systems for next generation gene therapies. ACS Synth Biol. 2017;6:1614-26
251. Givens BE, Naguib YW, Geary SM, Devor EJ, Salem AK. Nanoparticle-based delivery of CRISPR/Cas9 genome-editing therapeutics. AAPS J. 2018;20:108
252. Nihongaki Y, Yamamoto S, Kawano F, Suzuki H, Sato M. CRISPR-Cas9-based photoactivatable transcription system. Chem Bio. 2015;22:169-74
253. Zhou XX, Zou X, Chung HK, Gao Y, Liu Y, Qi LS. et al. A Single-chain photoswitchable CRISPR-Cas9 architecture for light-inducible gene editing and transcription. ACS Chem Biol. 2018;13:443-8
254. Shao J, Wang M, Yu G, Zhu S, Yu Y, Heng BC. et al. Synthetic far-red light-mediated CRISPR-dCas9 device for inducing functional neuronal differentiation. PNAS. 2018;115:E6722-E30
255. Wang P, Zhang L, Zheng W, Cong L, Guo Z, Xie Y. et al. Thermo-triggered release of CRISPR-Cas9 system by lipid-encapsulated gold nanoparticles for tumor therapy. Angew Chem Int Ed. 2018;57:1491-6
256. Ali MRK, Wu Y, Tang Y, Xiao H, Chen K, Han T. et al. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. PNAS. 2017;114:E5655-E63
257. Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13-22
258. Wang YX, Choi Y, Chen Z, Laurent S, Gibbs SL. Molecular imaging: from bench to clinic. Biomed Res Int. 2014;2014:357258
259. Goswami PP, Syed A, Beck CL, Albright TR, Mahoney KM, Unash R. et al. BODIPY-derived photoremovable protecting groups unmasked with green light. J Am Chem Soc. 2015;137:3783-6
260. Wu X, Lee H, Bilsel O, Zhang Y, Li Z, Chen T. et al. Tailoring dye-sensitized upconversion nanoparticle excitation bands towards excitation wavelength selective imaging. Nanoscale. 2015;7:18424-8
261. Resch-Genger U, Gorris HH. Perspectives and challenges of photon-upconversion nanoparticles - Part I: routes to brighter particles and quantitative spectroscopic studies. Anal Bioanal Chem. 2017;409:5855-74
262. Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112:2739-79
263. Fu C, Tan L, Ren X, Wu Q, Shao H, Ren J. et al. Interlayer expansion of 2D MoS2 nanosheets for highly improved photothermal therapy of tumors in vitro and in vivo. Chem Commun. 2018;54:13989-92
264. Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114:10869-939
265. Gohy JF, Zhao Y. Photo-responsive block copolymer micelles: design and behavior. Chem Soc Rev. 2013;42:7117-29
266. Zhang J, Guo W, Li Q, Wang Z, Liu S. The effects and the potential mechanism of environmental transformation of metal nanoparticles on their toxicity in organisms. Environ Sci Nano. 2018;5:2482-99
267. Kang H, Gravier J, Bao K, Wada H, Lee JH, Baek Y. et al. Renal clearable organic nanocarriers for bioimaging and drug delivery. Adv Mater. 2016;28:8162-8
268. Zhen X, Cheng P, Pu K. Recent advances in cell membrane-camouflaged nanoparticles for cancer phototherapy. Small. 2019;15:1804105
269. Wen J, Tian Y, Mei Z, Wu W, Tian Y. Synthesis of polypyrrole nanoparticles and their applications in electrically conductive adhesives for improving conductivity. RSC Adv. 2017;7:53219-25
270. Lv R, Wang D, Xiao L, Chen G, Xia J, Prasad PN. Stable ICG-loaded upconversion nanoparticles: silica core/shell theranostic nanoplatform for dual-modal upconversion and photoacoustic imaging together with photothermal therapy. Sci Rep. 2017;7:15753
271. Yue J, Feliciano TJ, Li W, Lee A, Odom TW. Gold nanoparticle size and shape effects on cellular uptake and intracellular distribution of siRNA nanoconstructs. Bioconjug Chem. 2017;28:1791-800
272. Morgan E, Wupperfeld D, Morales D, Reich N. Shape Matters: Gold nanoparticle shape impacts the biological activity of siRNA delivery. Bioconjug Chem. 2019;30:853-60
273. Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK. et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. PNAS. 1991;88:11460-4
274. Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220-33
275. Bunka DHJ, Stockley PG. Aptamers come of age-at last. Nat Rev Microbiol. 2006;4:588
276. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-87
277. Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B. et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8:745-52
278. Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. Nanofabricated particles for engineered drug therapies: a preliminary biodistribution study of PRINT nanoparticles. J Control Release. 2007;121:10-8
279. Li KC, Chu HC, Lin Y, Tuan HY, Hu YC. PEGylated copper nanowires as a novel photothermal therapy agent. ACS Appl Mater Interfaces. 2016;8:12082-90
Author contact
![]() Corresponding author: bengangedu.sg
Corresponding author: bengangedu.sg
 Global reach, higher impact
Global reach, higher impact