13.3
Impact Factor
Theranostics 2019; 9(11):3388-3397. doi:10.7150/thno.34031 This issue Cite
Research Paper
A versatile supramolecular nanoadjuvant that activates NF-κB for cancer immunotherapy
1. The First Affiliated Hospital, Biomedical Translational Research Institute and School of Pharmacy, Jinan University, Guangzhou 510632, China
2. School of Materials Science and Engineering, Center of Functional Biomaterials, Key Laboratory of Polymeric Composite Materials and Functional Materials of Ministry of Education, GD Research Center for Functional Biomaterials Engineering and Technology, Sun Yat-sen University, Guangzhou 510275, China
3. State Key Laboratory of Medicinal Chemical Biology, Key Laboratory of Bioactive Materials, Ministry of Education, College of Life Sciences, and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, P. R. China
Abstract
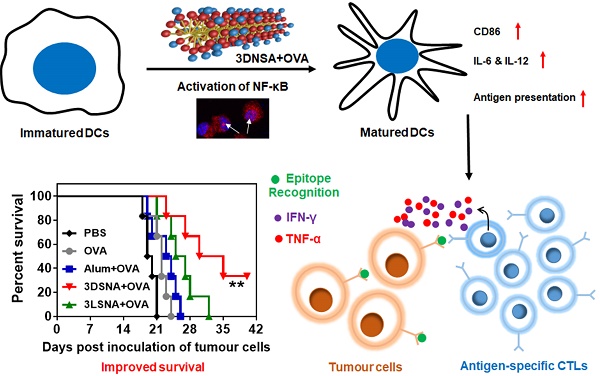
Although powerful adjuvants hold promise of vaccines for cancer immunotherapy, cumbersome preparation processes, elusive mechanisms and failure to induce T cell responses have largely limited their clinical translation. Due to their ease of synthesis, good biocompatibility and designable bioactivity, peptide derivatives-based supramolecular nanomaterials have attracted increasing interest in improving the immunogenicity of cancer vaccines.
Methods: Herein, we synthesized an NF-κB-activating supramolecular nanoadjuvant (3DSNA) that is prepared by pH-triggering self-assembly of a positively charged D-configurational peptide derivative. The immunostimulatory activity of 3DNSA was explored in vitro and in vivo.
Results: 3DSNA can strongly absorb the model antigen (ovalbumin, OVA) through electrostatic interaction. Then, 3DSNA promotes ingestion and cross-presentation of OVA, upregulation of costimulatory factors (CD80 and CD86) and secretion of proinflammatory cytokines (IL-6 and IL-12) by dendritic cells (DCs), accompanied by activation of the innate immune response (NF-κB signaling), resulting in long-term antigen-specific memory and effector CD8+ T cells response. When compared with conventional aluminum hydroxide adjuvant and the corresponding L-configurational supramolecular nanoadjuvant (3LSNA), 3DSNA-adjuvanted OVA (3DSNA+OVA) significantly prevents oncogenesis in naïve mice with a complete response rate of 60 %, restrains the tumor growth and prolongs the survival of melanoma-bearing mice.
Conclusion: These findings demonstrate that 3DSNA is a promising neo-adjuvant that enables various vaccines to be therapeutic for many important diseases including cancer.
Keywords: Adjuvant, peptide self-assembly, immunotherapy, NF-κB activation, anti-cancer
 Global reach, higher impact
Global reach, higher impact