13.3
Impact Factor
Theranostics 2019; 9(15):4391-4408. doi:10.7150/thno.32462 This issue Cite
Research Paper
ZnAs@SiO2 nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling
1. Department of Ultrasound, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province 519000, China
2. Center for Interventional Medicine, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province 519000, China
3. Guangdong Provincial Key Laboratory of Biomedical Imaging and Guangdong Provincial Engineering Research Center of Molecular Imaging, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province 519000, China
4. State Key Laboratory of Physical Chemistry of Solid Surfaces, The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, The Key Laboratory for Chemical Biology of Fujian Province, and Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian Province 361005, China
# These authors contributed equally to this article.
Abstract
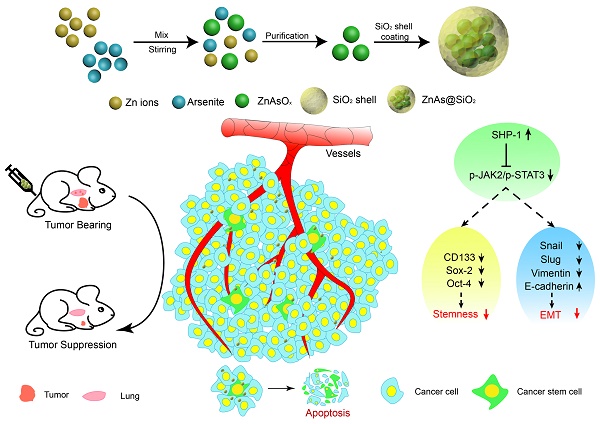
Rationale: Current therapies for hepatocellular carcinoma (HCC) are hampered by treatment failure and recurrence due to the remaining treatment-resistant liver cancer stem cells (CSCs). Stemness and epithelial-mesenchymal transition (EMT) are regarded as two fundamental characteristics of liver CSCs necessary for cancer progression; thus, drugs that simultaneously target both characteristics should prove effective in eliminating HCC and impeding recurrence. In this study, we developed new arsenic trioxide (ATO)-based nanoparticles (NPs), which are expected to be more effective than the current HCC therapy, and explored their potential mechanism.
Methods: A “one-pot” reverse emulsification approach was employed to prepare the ZnAs@SiO2 NPs. HCC cell lines, MHCC97L and Hep3b, were used to analyze the antitumor activity of ZnAs@SiO2 NPs in vitro and in vivo by quantifying cell growth and metastasis as well as to study the effect on stemness and EMT. SHP-1 siRNA was used to validate the role of the SHP-1/JAK2/STAT3 signaling pathway in mediating inhibition of stemness and EMT by ZnAs@SiO2.
Results: Compared with the current ATO treatment, ZnAs@SiO2 NPs promoted apoptosis and significantly inhibited proliferation, migration, and invasion of both MHCC97L and Hep3b cells. In the in vivo assay, ZnAs@SiO2 NPs inhibited tumor growth by 2.2-fold and metastasis by 3.5-fold as compared to ATO. The ZnAs@SiO2 NPs also inhibited tumor spheroid formation in vitro and tumor initiation in vivo and induced significant changes in the expression of stemness markers (CD133, Sox-2, and Oct-4) and EMT markers (E-cadherin, Vimentin, and Slug) both in vitro and in vivo. These effects of ZnAs@SiO2 that correlated with prognosis of HCC were mediated by the SHP-1/JAK2/STAT3 signaling.
Conclusions: ZnAs@SiO2 NPs can effectively suppress tumor initiation, growth, metastasis, and inhibit stemness and EMT through regulation of SHP-1/JAK2/STAT3 signaling pathway in liver cancer cells in vitro and in vivo. Thus, ZnAs@SiO2 NPs have immense potential for HCC treatment in the future.
Keywords: Arsenic trioxide nanoparticles, stemness, epithelial-mesenchymal transition, hepatocellular carcinoma, SHP-1
 Global reach, higher impact
Global reach, higher impact