13.3
Impact Factor
Theranostics 2019; 9(21):6063-6079. doi:10.7150/thno.36735 This issue Cite
Research Paper
Copy number gain of ZEB1 mediates a double-negative feedback loop with miR-33a-5p that regulates EMT and bone metastasis of prostate cancer dependent on TGF-β signaling
1. Department of Orthopaedic Surgery, the First Affiliated Hospital of Sun Yat-sen University, 510080, Guangzhou, China
2. Guangdong Provincial Key Laboratory of Orthopedics and Traumatology, 510080,Guangzhou, Guangdong Province, China
3. Clinical Experimental Center, Jiangmen Central Hospital, Affiliated Jiangmen Hospital of Sun Yat-sen University, Jiangmen, China
4. Department of radiology, the First Affiliated Hospital of Sun Yat-sen University, 510080, Guangzhou, China
5. Department of Pathology, the First Affiliated Hospital of Sun Yat-sen University, 510080, Guangzhou, China
6. Department of Pathology, the First People's Hospital of Guangzhou City, 510180, Guangzhou, Guangdong Province, China
#These authors contributed equally to this work
Abstract
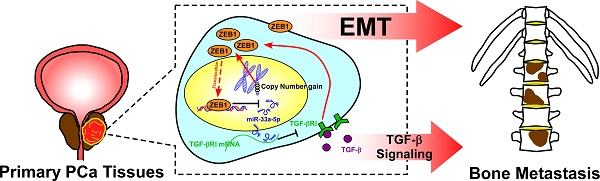
Background: The reciprocal repressive loop between ZEB1 and miRNAs has been extensively reported to play an important role in tumor progression and metastasis of various human tumor types. The aim of this study was to elucidate the role and the underlying mechanism of the double-negative feedback loop between ZEB1and miR-33a-5p in bone metastasis of prostate cancer (PCa).
Methods: miR-33a-5p expression was examined in 40 bone metastatic and 165 non-bone metastatic PCa tissues by real-time PCR. Statistical analysis was performed to evaluate the clinical correlation between miR-33a-5p expression and clinicopathological characteristics, and overall and bone metastasis-free survival in PCa patients. The biological roles of miR-33a-5p in bone metastasis of PCa were investigated both by EMT and the Transwell assay in vitro, and by a mouse model of left cardiac ventricle inoculation in vivo. siRNA library, real-time PCR and chromatin immunoprecipitation (ChIP) were used to identify the underlying mechanism responsible for the decreased expression of miR-33a-5p in PCa. Bioinformatics analysis, Western blotting and luciferase reporter analysis were employed to examine the relationship between miR-33a-5p and its potential targets. Clinical correlation of miR-33a-5p with its targets was examined in human PCa tissues and primary PCa cells.
Results: miR-33a-5p expression was downregulated in PCa tissues with bone metastasis and bone-derived cells, and low expression of miR-33a-5p strongly and positively correlated with advanced clinicopathological characteristics, and shorter overall and bone metastasis-free survival in PCa patients. Upregulating miR-33a-5p inhibited, while silencing miR-33a-5p promoted EMT, invasion and migration of PCa cells. Importantly, upregulating miR-33a-5p significantly repressed bone metastasis of PC-3 cells in vivo. Our results further revealed that recurrent ZEB1 upregulation induced by copy number gains transcriptionally inhibited miR-33a-5p expression, contributing to the reduced expression of miR-33a-5p in bone metastatic PCa tissues. In turn, miR-33a-5p formed a double negative feedback loop with ZEB1 in target-independent manner, which was dependent on TGF-β signaling. Finally, the clinical negative correlations of miR-33a-5p with ZEB1 expression and TGF-β signaling activity were demonstrated in PCa tissues and primary PCa cells.
Conclusion: Our findings elucidated that copy number gains of ZEB1-triggered a TGF-β signaling-dependent miR-33a-5p-mediated negative feedback loop was highly relevant to the bone metastasis of PCa.
Keywords: miR-33a-5p, ZEB1, double negative feedback loop, bone metastasis, prostate cancer
 Global reach, higher impact
Global reach, higher impact