13.3
Impact Factor
Theranostics 2019; 9(21):6269-6283. doi:10.7150/thno.37139 This issue Cite
Research Paper
Membrane TLR9 Positive Neutrophil Mediated MPLA Protects Against Fatal Bacterial Sepsis
1. Department of Molecular Biology, College of Basic Medical Sciences Jilin University, Changchun 130021, China
2. Department of Radiation Oncology, The University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
3. China-Japan Union Hospital, Jilin University, Changchun, Jilin 130021, China
4. Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA
5. Jinan University Institute of Tumor Pharmacology, Guangzhou 510632, China
6. Tumor Marker Research Center, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China
7. Department of Immunology, College of Basic Medical Sciences, Jilin University, Changchun 130021, China
8. Tumor Three Wards, Nanyang Central Hospital of Henan Province, Nanyang, Henan, 473000, China
9. Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
* These authors contributed equally to the work
Abstract
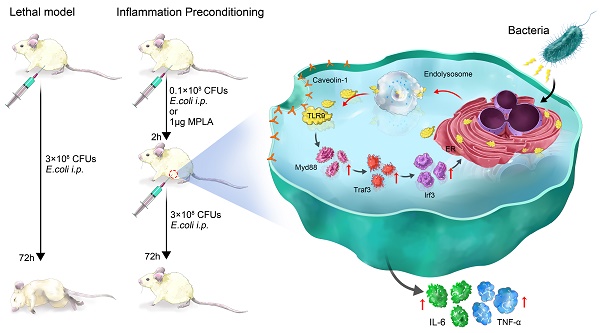
Sepsis is a major cause of patient mortality and morbidity from bacterial infections. Although neutrophils are known to be important in the development of sepsis, how distinctive neutrophil subtypes regulate inflammatory processes involved in septicemia remains unclear. Preconditioning protects organisms against subsequent higher-dose exposures to the same, or even different, stimuli. Several studies have reported various effects of preconditioning on immune cells. However, the detailed mechanisms underlying neutrophil-mediated protection through preconditioning in sepsis remain unknown.
Methods: Flow cytometry was conducted to sort the mice peritoneal lavage cells and the blood samples from patients with sepsis. Western blotting and ELISA were carried out to elucidate the expression of TLR9 signal transduction pathway proteins. Histological analysis was used to assess the effect of InP on intestine and liver structure in tlr9-/- and cav-1-/- mice. Fluorescence microscopy, Co-IP, and FRET were carried out to determine the association of TLR9 with Cav-1.
Results: We show that membrane toll-like receptor-9 positive (mTLR9+) neutrophils exert a protective effect against fatal bacterial infections through the process of inflammatory preconditioning (InP). InP, which occurs in the setting of a low-dose bacterial challenge, active ingredient is Monophosphoryl lipid A (MPLA), triggers the membrane translocation of TLR9 from the neutrophil cytosol, where it binds to Cav-1. Our findings showed that InP enables TLR9 to facilitate MyD88-mediated TRAF3 and IRF3 signal transduction. Depletion of either TLR9 or Cav-1 largely eliminates the neutrophil-mediated InP effect in sepsis models in vitro and in vivo. Further, examination of clinical samples from patients with sepsis showed that clinical outcomes and likelihood of recovery are closely correlated with mTLR9 and Cav-1 expression in circulating neutrophils.
Conclusion: These results demonstrate that the TLR9-Cav-1 axis is a critical signaling pathway involved in the regulation of neutrophil-dependent MPLA mediated InP, and the presence of mTLR9+ neutrophils could be an attractive indicator of clinical outcomes in bacterial sepsis that could be further explored as a potential therapeutic target.
Keywords: sepsis, neutrophils, TLR9, caveolin-1, preconditioning, MPLA
 Global reach, higher impact
Global reach, higher impact