13.3
Impact Factor
Theranostics 2019; 9(23):6764-6779. doi:10.7150/thno.36334 This issue Cite
Research Paper
Reversing activity of cancer associated fibroblast for staged glycolipid micelles against internal breast tumor cells
1. College of Pharmaceutical Science, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, People's Republic of China.
2. Ocean College, Zhejiang University, 1 Zheda Road, Zhoushan 316021, People's Republic of China.
3. The First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Road, Hangzhou 310058, China
Abstract
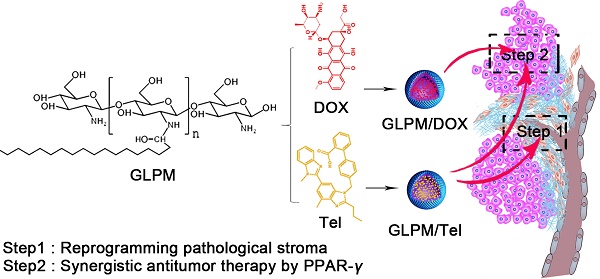
Rationale: Nano-carrier based combinational therapies for tumor cells hold great potential to improve the outcomes of patients. However, cancer associated fibroblasts (CAFs) in desmoplastic tumors and the derived pathological tumor stroma severely impede the access and sensitibity of tumor cells to antitumor therapies.
Methods: Glycolipid-based polymeric micelles (GLPM) were developed to encapsulate an angiotensin II receptor I inhibitor (telmisartan, Tel) and a cytotoxic drug (doxorubicin, DOX) respectively, which could exert combinational antitumor efficacy by reprogramming tumor microenvironment to expose the vulnerability of internal tumor cells.
Results: As demonstrated, α-SMA positive CAFs significantly decreased after the pre-administration of GLPM/Tel in vitro, which accordingly inhibited the secretion of the CAFs derived stroma. The tumor vessels were further decompressed as a result of the alleviated solid stress inside the tumor masses, which promoted more intratumoral drug delivery and penetration. Ultimately, staged administration of the combined GLPM/Tel and GLPM/DOX at the screened molar ratio not only inhibited the stroma continuously, but also achieved a synergistic antitumor effect through the apoptosis-related peroxisome proliferator-activated receptor-gamma (PPAR-γ) pathway.
Conclusion: In summary, the strategy of suppressing tumor stroma for subsequent combinational therapies against internal breast tumor cells could provide avenues for management of intractable desmoplastic tumors.
Keywords: cancer associated fibroblasts, stroma, glycolipid micelles, doxorubicin, telmisartan.
 Global reach, higher impact
Global reach, higher impact