13.3
Impact Factor
Theranostics 2019; 9(25):7583-7598. doi:10.7150/thno.37717 This issue Cite
Research Paper
HBX-induced miR-5188 impairs FOXO1 to stimulate β-catenin nuclear translocation and promotes tumor stemness in hepatocellular carcinoma
1. Affiliated Cancer Hospital & Institute of Guangzhou Medical University; Laboratory of Protein Modification and Degradation, State Key Laboratory of Respiratory Disease, Guangzhou Medical University, Guangzhou, China.
2. Cancer Institute, School of Basic Medical Science, Southern Medical University, Guangzhou, Guangdong, China.
3. Cancer Center, Integrated Hospital of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong, China.
4. The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China.
5. Department of Oncology, The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China.
6. Brain Hospital of Hunan Province, Changsha, Hunan, China.
7. Department of Gastroenterology, Hunan People's Hospital, Changsha, Hunan, China.
*These authors contributed equally to this work.
Abstract
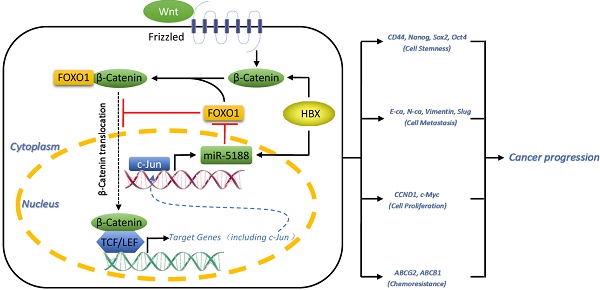
Cancer stem cells (CSCs) are the key factor in determining cancer recurrence, metastasis, chemoresistance and patient prognosis in hepatocellular carcinoma (HCC). The role of miR-5188 in cancer stemness has never been documented. In this study, we investigated the clinical and biological roles of miR-5188 in HCC. Methods: MiRNA expression in HCC was analyzed by bioinformatics analysis and in situ hybridization. The biological effect of miR-5188 was demonstrated in both in vitro and in vivo studies through the ectopic expression of miR-5188. The target gene and molecular pathway of miR-5188 were characterized using bioinformatics tools, dual-luciferase reporter assays, gene knockdown, and rescue experiments. Results: MiR-5188 was shown to be upregulated and confer poor prognosis in HCC patient data from TCGA database. MiR-5188 was subsequently identified as a significant inducer of cancer stemness that promotes HCC pathogenesis. Specifically, the targeting of miR-5188 by its antagomir markedly prolonged the survival time of HCC-bearing mice and improved HCC cell chemosensitivity in vivo. Mechanistic analysis indicated that miR-5188 directly targets FOXO1, which interacts with β-catenin in the cytoplasm to reduce the nuclear translocation of β-catenin and promotes the activation of Wnt signaling and downstream tumor stemness, EMT, and c-Jun. Moreover, c-Jun transcriptionally activates miR-5188 expression, forming a positive feedback loop. Interestingly, the miR-5188-FOXO1/β-catenin-c-Jun feedback loop was induced by hepatitis X protein (HBX) through Wnt signaling and participated in the HBX-induced pathogenesis of HCC. Finally, analyses of transcriptomics data and our clinical data supported the significance of the abnormal expression of the miR-5188 pathway in HCC pathogenesis. Conclusions: These findings present the inhibition of miR-5188 as a novel strategy for the efficient elimination of CSCs to prevent tumor metastasis, recurrence and chemoresistance in patients with hepatocellular carcinoma. Our study highlights the importance of miR-5188 as a tumor stemness inducer that acts as a potential target for HCC treatment.
Keywords: MiR-5188, Hepatitis X protein, Cancer stem cells, Hepatocellular carcinoma
 Global reach, higher impact
Global reach, higher impact