13.3
Impact Factor
Theranostics 2019; 9(25):7616-7627. doi:10.7150/thno.35900 This issue Cite
Research Paper
A novel Granzyme B nanoparticle delivery system simulates immune cell functions for suppression of solid tumors
1. Department of Medical Laboratory, School of Medical Technology, Tianjin Medical University, Tianjin 300203, China
2. 3rd Department of Breast Cancer, China Tianjin Breast Cancer Prevention, Treatment and Research center, Tianjin Medical University Cancer Institute and Hospital, Tianjin 300060, China
3. Tianjin Key Laboratory of Composite and Functional Materials, School of Materials Science & Engineering, Tianjin University, Tianjin 300072, China
4. Department of Chemical and Biomolecular Engineering, University of California, Los Angeles, CA 90095, USA
5. Laboratory of Neuro-Oncology, Tianjin Neurological Institute, Department of Neurosurgery, Tianjin Medical University General Hospital and Key Laboratory of Neurotrauma, Variation, and Regeneration, Ministry of Education and Tianjin Municipal Government, Tianjin 300052, China
Abstract
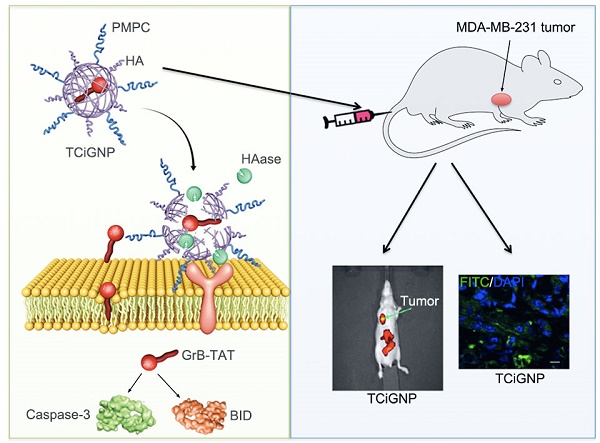
Cell-based immunotherapy for the treatment of hematologic malignancies, such as leukemia and lymphoma, has seen much success and played an increasingly important role in clinical studies. Nevertheless, the efficacy of immunotherapy in solid tumors still needs improvements due to the immunosuppressive properties of tumor cells and the microenvironment. To overcome these limitations, we prepared a novel tumor-targeting delivery system based on the underlying mechanism of immune-targeted cell death that encapsulated granzyme B protein within a porous polymeric nanocapsule.
Methods: A cell-penetrating peptide TAT was attached onto granzyme B (GrB) to enhance its transmembrane transport efficiency and potency to induce cell apoptosis. The endocytosis and internalization pathways of GrB-TAT (GrB-T) were analyzed in comparison with perforin by confocal microscopy and flow cytometry. Furthermore, the positively charged GrB-T was wrapped into nanoparticles by p-2-methacryloyloxy ethyl phosphorylcholine (PMPC)-modified HA (hyaluronic acid). The nanoparticles (called TCiGNPs) were characterized in terms of zeta potential and by transmission electron microscopy (TEM). The in vitro anti-tumor effects of GrB-T were examined by cell apoptosis assay and Western blotting analysis. The in vivo anti-tumor therapeutic efficacy of TCiGNPs was evaluated in a mouse tumor model.
Results: The TAT peptide could play a role similar to perforin to mediate direct transmembrane transfer of GrB and improve GrB-induced cell apoptosis. The TCiGNPs were successfully synthesized and accumulated in the solid tumor through enhanced permeability and retention (EPR) effect. In the tumor microenvironment, TCiGNPs could be degraded by hyaluronidase and triggered the release of GrB-T. The TAT peptide enabled the translocation of GrB across the plasma membrane to induce tumor cell apoptosis in vivo.
Conclusion: We successfully developed a granzyme B delivery system with a GrB-T core and a PMPC/HA shell that simulated CTL/NK cell-mediated cancer immunotherapy mechanism. The GrB delivery system holds great promise for cancer treatment analogous to the CTL/NK cell-induced immunotherapy.
Keywords: Granzyme B delivery, tumor therapy, nanoparticles, biomimetics
 Global reach, higher impact
Global reach, higher impact