13.3
Impact Factor
Theranostics 2019; 9(26):8073-8090. doi:10.7150/thno.37198 This issue Cite
Review
Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment
1. Center for Theragnosis, Biomedical Research Institute, Korea Institute of Science and Technology (KIST), Hwarang-ro 14-gil 5, Seongbuk-gu, Seoul 02792, Republic of Korea
2. KU-KIST Graduate School of Converging Science and Technology, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Republic of Korea
3. Department of Cancer Biology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, Massachusetts 02215, United States
¶These authors contributed equally to this work
Received 2019-6-1; Accepted 2019-9-16; Published 2019-10-17
Abstract
The use of nanomedicine for cancer treatment takes advantage of its preferential accumulation in tumors owing to the enhanced permeability and retention (EPR) effect. The development of cancer nanomedicine has promised highly effective treatment options unprecedented by standard therapeutics. However, the therapeutic efficacy of passively targeted nanomedicine is not always satisfactory because it is largely influenced by the heterogeneity of the intensity of the EPR effect exhibited within a tumor, at different stages of a tumor, and among individual tumors. In addition, limited data on EPR effectiveness in human hinders further clinical translation of nanomedicine. This unsatisfactory therapeutic outcome in mice and humans necessitates novel approaches to improve the EPR effect. This review focuses on current attempts at overcoming the limitations of traditional EPR-dependent nanomedicine by incorporating supplementary strategies, such as additional molecular targeting, physical alteration, or physiological remodeling of the tumor microenvironment. This review will provide valuable insight to researchers who seek to overcome the limitations of relying on the EPR effect alone in cancer nanomedicine and go “beyond the EPR effect”.
Keywords: EPR effect, targeted therapy, nanoparticle, cancer treatment, drug delivery
1. Introduction
The targeted delivery of therapeutics for cancer treatment has been developed alongside the progress of nanotechnology [1, 2]. Nanoparticles (NPs) that incorporate one or more cancer drugs, such as chemotherapeutic agents, molecularly targeted drugs, and small interfering RNAs, are intravenously administered for delivery to tumors [3]. They are designed to prolong systemic circulation, minimize any off-target interactions, and improve the target-specific accumulation of therapeutics. Although the surface of NPs may be embellished with specific tumor-targeting molecules to induce more “active” targeting, the majority of the current targeting strategies is “passive”, meaning that delivery relies solely on the unique physical characteristics of NPs and tumors [4-6]. When tumors form, their rapid growth results in neovasculature with wide fenestrations and suppressed lymphatic drainage [7]. Consequently, nanoscale drug carriers that do not readily extravasate into normal healthy tissues effectively pass through the leaky blood vessels of the tumor and accumulate within the target site. This preferential accumulation of NPs in tumors is known as the enhanced permeability and retention (EPR) effect [4, 8]. This approach has shown many promising results exceeding those of standard therapeutics, including reduced toxicity in healthy tissues and increased drug concentration in the target site. In fact, multiple nanomedicine carriers, such as liposomes, micelles, albumin NPs, and polymer conjugates, have been approved for the treatment of various cancers over the past 20 years [9].
As the field of cancer nanomedicine rapidly progresses, it has become evident that the therapeutic efficacy of passively targeted nanomedicine is immensely influenced by the heterogeneity of the intensity of the EPR effect within a tumor, at different stages of a tumor, and among individual tumors [10]. Tumors are heterogeneous by nature and various studies have shown that the EPR effect within tumors is also highly heterogeneous [11-13]. Heterogeneity is present among tumor models of different species, diverse tumor types of the same origin, tumors at different locations in the same patient, and even at different stages of the same tumor during its development [14-16]. Within a tumor, variations in the thickness and density of the extracellular matrix (ECM), uneven blood flow distribution, and disproportionate vessel permeability have been shown to affect the heterogeneity of the EPR effect [11, 17]. These factors vary substantially among tumors and even within the same tumor over time. In addition, the physicochemical properties of a nanoscale drug carrier, such as size, shape, and elasticity, contribute to the heterogeneity of the EPR effect. Several studies have shown that the size, shape, and elasticity of nanomedicine carriers impacted extravasation from the vessel and retention in the tumor site, resulting in varied therapeutic efficacy [18-20]. However, it is critical to mention that our current understanding of the EPR effect is mostly based on animal data, especially from fast-growing xenograft models in mice, which have been used most to explain the EPR effect. Experimental data on EPR effectiveness in patients should be further accumulated for the successful clinical use of nanomedicine.
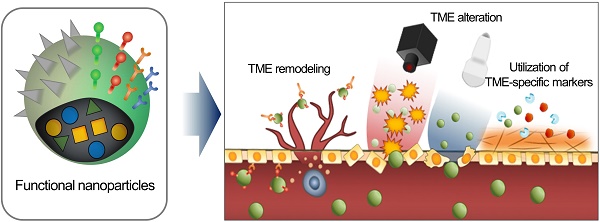
Consequently, the delivery of an EPR-dependent nanomedicine can be further enhanced to maximize its therapeutic efficacy [21, 22]. Although the benefits of nanomedicine in principle surpass the efficacy of standard therapeutics, its therapeutic efficacy is not always satisfactory because if it is largely influenced by the heterogeneity of the intensity of the EPR effect [23-25]. This unsatisfactory therapeutic outcome in mice and humans requires novel approaches to improve the EPR effect by additional different strategies (Figure 1). First, tumor microenvironment (TME)-specific molecular markers including ECM components, tumor-specific pathophysiological conditions, and TME-specific enzymes, can be utilized with nanomedicine. Second, physical alteration of TME by photodynamic, sonodynamic, and radiation therapies can be applied to improve the EPR effect. Third, the physiological remodeling of TME aims to improve the EPR effect indirectly by inducing artificial TME or promoting vascular remodeling. In this review, we summarize three different combination strategies based on their enhancement of the EPR effect to maximize the therapeutic benefits of nanomedicine, focusing on the utilization of TME-specific molecular markers, alteration of TME aided by external sources and physiological remodeling of TME.
Schematic illustration of three synergistically combined strategies (utilization of TME-specific molecular markers, alteration of TME aided by external sources and physiological remodeling of TME) to improve the EPR effect on the various factors in TME.
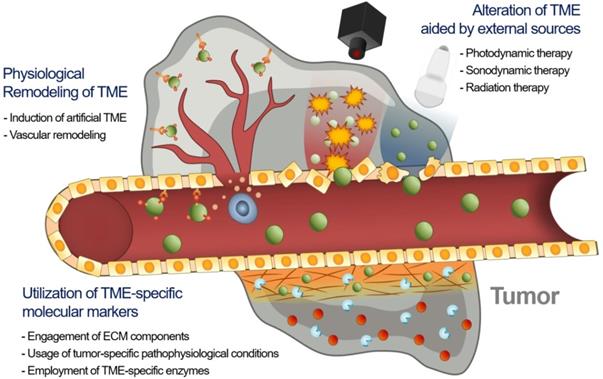
Recent advances in synergistically combined strategies to improve the EPR effect in TME
| Class | Type | Target | Material(composition) | Brief description | Tumor model | Ref |
|---|---|---|---|---|---|---|
| Utilization of TME-specific molecular markers | ECM | CD44 receptor | HA | Thermosensitive self-assembled NPs with HA/PTX | 4T1 | [42] |
| ECM | EGFR | HA | Dual-targeting strategy with low toxicity | HCCLM3 | [44] | |
| Enzyme | Cathepsin B | Peptide (FRRG) | Self-assembling carrier-free NPs of prodrug containing DOX | HT-29 | [66] | |
| Enzyme | MMP-2 | Liposome with sodium bicarbonate | Nanoscale micelle systems binding EGFR/HER2 complex | 4T1 | [64] | |
| Alteration of TME aided by external sources | PDT | Pgp | Doxil and Abraxane | Depleting MDR cancer cells by PDT using APCs and Doxil® | KB, 3T3 | [82] |
| PDT | Light-induced 1O2 | Ce6, thioketal linker | ROS-responsive Ce6/DOX-loaded RHPPE NPs for PDT | MCF-7/ADR | [83] | |
| PDT | Light-induced 1O2 | RTP/LDH nanohybrids | NIR activated supramolecular photosensitizers for two-photon PDT | HeLa | [84] | |
| SDT | Transferrin receptor | Protoporphyrin IX | Nanosonosensitizers for ROS-mediated SDT | HeLa | [85] | |
| RT | αvβ3 integrin | AuNPs, cRGD | Sequential chemotherapy after RT using vascular-targeted AuNPs | Sarcoma | [92] | |
| Physiological remodeling of TME | ECM | Cathepsin B | Peptide (KGRR) | Metabolic precursor for tumor-specific fluorescence imaging | HT-29 | [101] |
| Enzyme | Caspase-3/-7 | Peptide (KGDEVD) | Metabolic precursor for tumor bioorthogonal apoptosis tracking | PC-3 | [146] | |
| Vascular | GSH | NO | NO therapy together with IR780 and PTX-loaded NPs | 4T1 | [113] | |
| Vascular | VEGF | PolysiRNA | Combination treatment with metronomic DOX and RNA interference NPs | PC-3 | [122] | |
| Vascular | Tubulin | Vascular disrupting agent (CKD-516) | Ischemia and necrosis inducing VDA in combination with DOX | VX2 | [147] | |
| Vascular | RhoA/ROCK | lysophosphatidic acid receptor 4 | Vascular network formation for chemo- and immunotherapy | GL261 | [127] | |
| ECM | Integrin | Peptide (RGD and YIGSR) | Transformable artificial ECM for tumor invasion and metastasis | MDA-MB-231 | [102] | |
| Multifunctional strategies targeting TME | Enzyme | Caspase-3 | Peptide (DEVD) | Radiation-induced apoptosis-targeted chemotherapy | C3H/HeN | [70] |
| Vascular | VEGF | Anginex and Avastin | Vessel normalization by angiogenesis inhibitor with RT | MA148, B16F10, SCK | [148] | |
| ECM | TAMs | DOX and Taxol | Chemotherapy combined with PDT by TME-remodeling TAMs | 4T1 | [135] |
APCs: antibody-photosensitizer conjugates, AuNPs: gold nanoparticles, GSH: glutathione, HER2: human epidermal growth factor receptor 2, LDH: layered double hydroxide, MMP-2: matrix metalloproteinase-2, RhoA/ROCK: Ras homolog gene family, member A/ Rho-associated protein kinase, RHPPE: SO-responsive PEGylated hyperbranched polyphosphates, RTP: room temperature phosphorescence
2. Utilization of TME-specific molecular markers
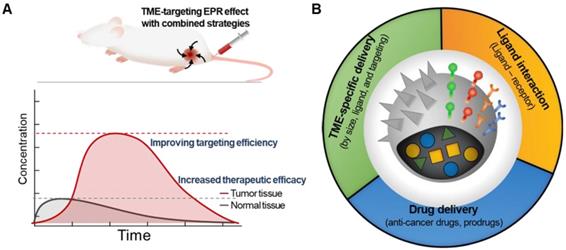
A prominent strategy to enhance the permeation and retention of nanomedicine in tumors, in addition to targeting the tumor cells themselves, is to add a moiety that specifically binds to components of the TME [26]. Targeting tumor cells by adding tumor cell-specific binding molecules has been a very popular strategy to overcome the limitations of traditional nanomedicine [27]. Since components of TME share many characteristics specific to them, targeting TME with chemical ligands has shown great potential in improving targeting efficiency in drug delivery and enhancing the therapeutic efficacy of cancer treatment (Figure 2A) [20, 28, 29]. Decorating NPs with TME-targeting ligands is one of the most effective ways to provide nanomedicine with TME-specific targeting ability to overcome tumor heterogeneity, considering that human patients possess remarkably diverse TMEs and that the physiological barriers to macromolecule delivery cannot be simply overcome by using the traditional EPR strategy alone (Figure 2B). The traditional EPR effect of NPs has been limited in clinical use due to unpredictable therapeutic efficiency, low delivery efficacy, and poor clinical outcomes.
2.1. Engagement of ECM components
It is clear that the EPR effect is highly associated with the physiological condition and heterogeneity of ECM at the tumor site [30]. Potential molecular targets in ECM that could be used to increase the EPR effect exerted on drug delivery systems (DDS) include fibrous ECM proteins, proteoglycan, growth factor receptors, and transmembrane receptors. A dense TME composed of fibrous proteins, such as collagen and fibronectin, directly reduces the vascular transport of nanomedicine [12]. Targeting and breaking down these molecules to loosen the ECM facilitates the entry of nanomedicine to the tumor site.
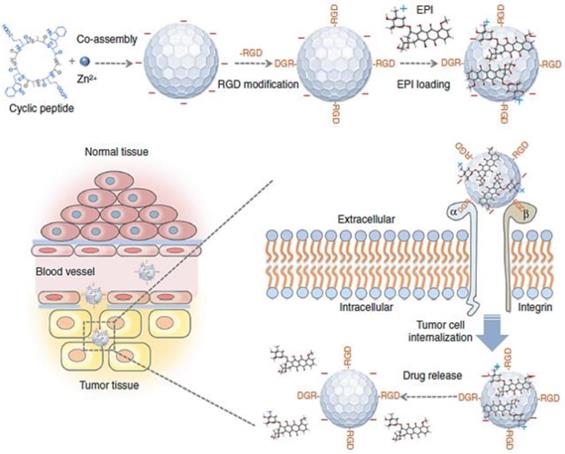
Among various transmembrane factors, integrin has been well established as a tumor-specific marker of angiogenic activity in ECM [31]. Integrin αvβ3, which is overexpressed on angiogenic vessels, plays a key role in tumor proliferation and metastasis. Targeting integrin αvβ3 leads NPs straight to the angiogenic vessels, increasing the extravasation of the NPs at the tumor site [32]. The Arg-Gly-Asp (RGD) peptide can recognize integrin αvβ3 and has therefore been widely used in anti-cancer research to increase the accumulation of macromolecules for the last two decades [33, 34]. For example, near-infrared (NIR) fluorescent NPs assembled by multiple cyclic peptides, cyclo[-(D-Ala-L-Glu-D-Ala-L-Trp)2-] were modified with RGD moieties and loaded with the chemotherapeutic agent, epirubicin (EPI). They showed increased accumulation in tumor tissue compared to nontumor tissues because of direct binding to integrin αvβ3 in addition to the EPR effect, resulting in enhanced therapeutic efficacy (Figure 3) [35]. Interestingly, the self-assembled NPs modified with RGD exhibited significantly reduced cardiotoxicity due to the specific delivery to the tumor site. Although RGD-based tumor ECM delivery has great potential to enhance the EPR effect of nanomedicine, some studies have questioned whether RGD-based peptide modification can induce serious toxicity. Indeed, it has been clearly demonstrated that peptide modification with the RGD moiety on NPs could induce severe toxicity associated with immune stimulation [36]. Interestingly, this unwanted immune response could be overcome by loading cytotoxic agents in the carrier [36]. According to Wang et al., the major immunotoxicity of cyclic RGD peptides in liposomes could not be eliminated by minimizing the amount of the peptide. However, encapsulation of cytotoxic agent (doxorubicin; DOX) shut off the lethal reaction and unwanted immunotoxicity, completely eliminating the unintended lethal IgG response in the body. This result showed the importance of precise control over the formulation of ECM-targeting peptide-based NPs at the development stage to minimize side effects and toxicity. These results appear to have important implications for the clinical application and therapeutic use of peptide modified NPs in the clinic for the optimized EPR effect. Considering the current low clinical use of NPs, these studies serve as valuable references to develop a successful clinically applicable NP with the effective EPR effect for patients.
Receptors that overexpressed in cancer cells, such as epidermal growth factor receptor (EGFR), have been investigated as binding sites for the targeted delivery of anti-cancer drugs. EGFR is often overexpressed in many types of cancers, and is involved in signaling pathways to regulate cell proliferation, differentiation, and inhibition of apoptosis [37]. The selection of targeting ligands on the surface of nanomedicine is critical in the design of formulations for EGFR targeting. Most types of ligands used for targeting EGFR have been monoclonal antibodies or fragments thereof, such as cetuximab, trastuzumab or panitumumab. Recently, endogenous ligands such as epidermal growth factor (EGF) have shown great potential for targeting EGFR on tumor cells. Because EGF has a smaller molecular weight (~ 6 kDa) than antibodies, targeting EGFR in TME offers unique advantages: the targeted nanomedicine penetrates more deeply into the tumor, and more rapid in vivo clearance can be facilitated [38]. Zalba et al. developed EGFR-targeted liposomes coupled with EGF for selective delivery of anti-cancer drugs, oxaliplatin into tumors. EGF-conjugated liposomes significantly decreased the IC50 of oxaliplatin in EGFR-positive colorectal cancer cell lines without enhancing the cytotoxicity of oxaliplatin in EGFR-negative colorectal cancer cell lines [39]. Interestingly, unlike free EGF, EGF coupled on the surface of the liposomes was not able to activate EGFR by EGF-EGFR docking. Therefore, EGFR in TME can be successfully targeted to improve the conventional EPR effect of NPs.
The conventional EPR effect usually resulted in an increased amount of nanomedicine in the tumor tissue. (A) Synergistic strategies can further enhance the accumulation of nanomedicine at the tumor site, indicating improved efficacy. (B) Various attempts to overcome the limitations of traditional EPR-reliant nanomedicine by incorporating additional molecular modification on NPs.
Schematic illustration of coassembled NPs with Zn2+ ions, cyclic peptides, epirubicin (EPI) and an RGD moiety show that they can accumulate in the tumor tissue by the EPR effect. Delivery tends to be increased by binding to the overexpressed αvβ3 integrin and tumor cell internalization. Adapted with permission from [35], copyright 2018 Nature Publishing Group.
Hyaluronan-based macromolecules that target CD44 receptors have been used to improve the targeting of ECM around tumors and present therapeutic potential in a number of cancers [40]. CD44 is often upregulated in various cancer cells and can be a target for hyaluronic acid (HA), which is one of the main components of ECM. HA-based NPs could preferentially accumulate at the tumor site via the EPR effect by binding to CD44. The significance of increasing the EPR effect with additional targeting molecules was clearly shown in a recent study by Liu et al. [41]. They modulated both the size of HA-based NPs and the loading components to synergistically suppress tumor growth and increase NPs accumulation in tumors. HA-shielded 200 nm NPs displayed an optimal EPR effect in 4T1 mouse breast tumor-bearing mice by achieving enhanced tissue penetration, suppressing the growth of primary tumor by 95%, and inhibiting tumor metastasis by 90%. In addition, Yaqi et al. developed a thermosensitive self-assembled nanoplatform that codelivers a matrix metalloproteinase inhibitor (marimastat) and HA-conjugated paclitaxel (PTX) prodrug for dual targeting of the TME and tumor cells. This combination promoted drug accumulation at tumor, tumor growth inhibition (12-fold, compared with the PTX-treated group), and metastasis inhibition (100%, compared with the control group), indicating that the combination of TME-targeting a HA-based nanomedicine with TME modulator is a highly promising strategy for cancer treatment [42]. To enhance the EPR effect, HA-based cell-penetrating peptide-modified lipid NPs were also prepared and evaluated in hepatocellular carcinoma cells. These NPs effectively penetrated the ECM and accumulated in the tumor due to the enhanced EPR effect, as demonstrated by low-intensity focused ultrasound (LIFU) imaging. Furthermore, dual targeting strategies with EGFR and CD44 have recently become a focus of research for the EPR effect enhancement [43, 44]. Although the dual combination of EGFR and HA targeting has not yet been widely studied, it has appeared as an efficacious way for tumor-targeted therapy to decrease the uncertainty of single targeting. In addition, PEGylated recombinant human hyaluronidase (PEGPH20) has been utilized to eliminate HA in TME. PEGPH20 has shown increased therapeutic effects in vivo, inhibiting tumor progression and metastasis. Thus, HA-degradable PEGPH20 has been investigated in combination with anti-cancer agents including gemcitabine, and has shown an increased therapeutic index in animal models [45, 46]. It is currently undergoing evaluation in phase III global clinical trial for treatment of pancreatic cancer patients [47].
2.2. Usage of tumor-specific pathophysiological conditions
As one of the characteristics of fast-growing malignant tumors, hypoxia is the common result of imbalance between the abnormal blood vessels and the high demand for nutrients and/or oxygen [48]. Hypoxia is known to be closely associated with tumor angiogenesis and metastasis, and it can lead to multi-drug resistance (MDR). Nanotechnology has an advantage in treating hypoxic conditions because the unique low oxygen environment provides an opportunity for stimuli-targeting NPs, and the poor angiogenesis can enhance the extravasation of macromolecules in the tumor tissues [23, 49]. To increase the EPR effect, NPs can be designed to bind directly to hypoxia-specific molecular markers, to reduce hypoxia-specific gene expression through small interfering RNA (siRNA) treatment, or to release cytotoxic drugs specifically under hypoxic conditions. As a hypoxia-specific molecular marker, increased expression of phosphatidylserine, a phenomenon normally associated with apoptosis, is observed on the external layer of hypoxic tumor cells and tumor-associated endothelial cell membranes. For example, Saposin C, a lysosomal protein that binds to phosphatidylserine, has been utilized to build NPs that bind to the hypoxic TME [50, 51]. The resulting NPs showed impressive therapeutic efficacy in the glioblastoma model, crossing the blood-brain barrier, exhibiting specific retention in the tumor tissue, and sensitizing the hypoxic cells.
Another application of nanotechnology that takes advantage of the intratumoral hypoxic conditions is delivering a siRNA that targets a hypoxia-related gene, hypoxia-inducible factor-1α (HIF-1α), which is transcribed into HIF-1α protein. HIF-1α is deeply associated with the activation of a series of genes that aggravate the tumor condition, such as promoting cell proliferation, migration, angiogenesis, low pH, and MDR [52]. A cationic micellar NP that incorporates HIF-1α siRNA (NP/siHIF nanocomplex) not only efficiently reduced tumor cell proliferation, migration, and angiogenesis in vitro, but also showed tumor growth inhibition and reduced MDR1 gene expression in vivo upon systemic administration [53, 54]. Perche et al. further utilized the hypoxic condition and developed a hypoxia-responsive copolymer that specifically exposes siRNA cargo to hypoxic tumor cells [55]. They developed a lipid-conjugated, polyethylene glycol (PEG)-shielded polyethyleneimine (PEI) nanocomplex with a hypoxia-sensitive linker (azobenzene; nitroimidazole derivative) and anti-green fluorescent protein siRNA. Under hypoxic conditions, the azobenzene linker is able to be degraded to release the protective PEG layer, thereby exposing the siRNA to the hypoxic tumor tissue and enabling its hypoxia-dependent cellular uptake into A549 tumor cells (spheroids). PEI is one of the most widely studied and the most successful cationic polymers for delivery of nucleic acid including siRNA. However, high molecular weight PEI has not only exhibited high transfection efficiency but also shown significant systemic toxicity. To reduce the potential toxicity of PEI-based delivery system, PEI has been mixed with other polymers, especially PEG. These various efforts have led to the successful clinical application of PEI to deliver vulnerable genetic biomaterials [56]. Similar to the azobenzene linker, 2-nitroimidazole has been widely utilized for hypoxia-sensitive drug delivery. Hydrophobic 2-nitroimidazole is converted to hydrophilic 2-aminoimidazole via a series of reductive reaction under hypoxic conditions. Thambi et al. introduced hydrophobic 2-nitroimidazole on the surface of carboxymethyl dextran-based NPs and loaded chemotherapeutic agents, DOX, within NPs [57]. The hypoxia-responsive NPs bearing DOX disassembled and released DOX under hypoxic conditions. These NPs demonstrated a high level of tumor-specific accumulation and delayed tumor progression upon systemic administration. Hypoxia-specific nanomedicines allow systemic administration at high doses due to their enhanced therapeutic efficacy and low toxicity. However, the extent of hypoxia varies significantly between tumors and even within a single tumor, and cells experiencing severe hypoxia represent a small population in most solid tumors. To overcome the heterogeneity of hypoxia and achieve the optimal therapeutic effect, artificial induction of hypoxic stress or combination with other existing therapies has also been successfully attempted [58]. Stimuli‐responsive DDS provide a great potential for innovative NPs development, triggering a series of synergistic therapeutic effects in the tumor site.
An emerging TME-targeting strategy of utilizing the acidity of TME has been well recognized in designing tumor-specific NPs. Many researchers have considered the acidity of TME as a potential therapeutic target since Otto Heinrich Warburg discovered the pathophysiological acidic condition of tumors as a result of lactate overproduction [59, 60]. Therapeutic strategies targeting tumor acidity have demonstrated that it can markedly enhance the tumor-specific accumulation and internalization of macromolecules. The acidic extracellular TME reverses the surface charge of NPs, which can lead to NP aggregation in the TME. Therefore, pH-sensitive NPs would ultimately enhance the preferential retention and accumulation of drugs in tumors by relying on the difference in pH between TME and normal tissues [61-63]. Modulation of the pH in TME by NPs and chemical agents can increase the uptake and therapeutic activity of nanomedicines, improving the treatment outcome. For example, treatment of murine 4T1 breast tumor-bearing mice with liposomal DOX and sodium bicarbonate showed 21-fold increase of drug uptake in the tumor site (compared to free nonliposomal bicarbonate) 24 h postinjection [64]. These results showed that modulating the acidity of TME holds great potential for conditioning TME towards improved therapeutic activity. These approaches can provide valuable insight into the development of nanomedicine to achieve improved uptake and retention.
2.3. Employment of TME-specific enzymes
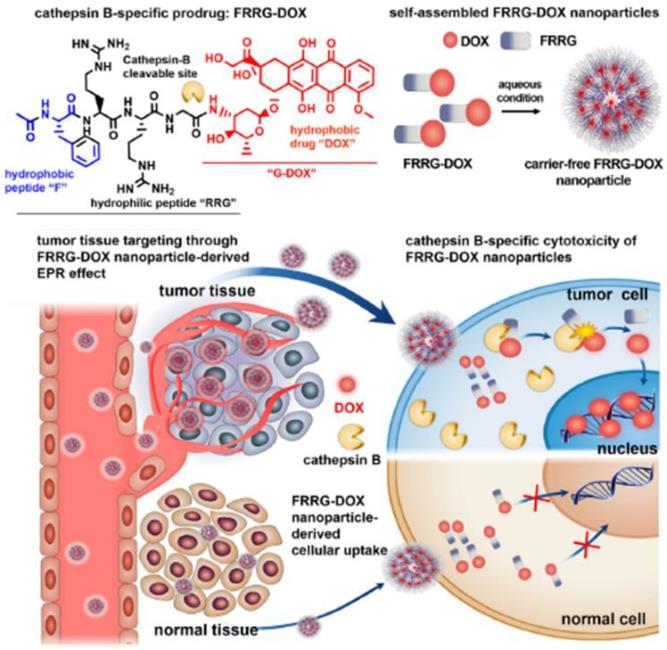
TME-specific enzymes can be used in several ways to activate the prodrug in DDS for anti-cancer therapy. This approach has several advantages including tumor-specific accumulation, low systemic toxicity, and high therapeutic efficacy. Enzyme-specific prodrug-based nanomedicine can provide a path forward to enable delivery to tumors for clinical use. The US Food and Drug Administration (FDA) has approved 30 prodrugs during the last decade, which accounts for more than the total number of approved nanomedicine products [65]. Additionally, 17% of new chemical FDA-approved drugs have been prodrug types over the past 3 years [65]. Clinical challenges and considerations in NPs development can overcome by novel prodrug strategies, solving the current clinical problem of the biopharmaceutical performance of NPs. Prodrug modification may be useful to decrease the critical limitations of NPs in terms of absorption, distribution, metabolism and excretion, which can contribute to their successful clinical application. Implementing a prodrug strategy in early NPs development would accelerate the clinical use and commercialization of nanomedicine. From this point of view, nanoscale self-assembled prodrugs, which exhibit high potential with simple structures and remarkable efficacy, have been extensively evaluated in various recent studies. For example, one of these prodrugs, consisting of peptide and DOX with an overall molecular weight of less than 2000 Da, can self-assemble to form NPs and accumulate in tumors by the EPR effect. It can then be activated by cathepsin B, an enzyme characteristically overexpressed in tumors, releasing free DOX in tumor (Figure 4) [66]. This approach is important because it can potentially overcome problems NPs currently faced, such as low drug loading efficiency (<10%) and difficulty in mass production. These problems have hindered successful commercialization of novel and functional NPs [67]. Most polymer-based nanomedicines with a sound strategy have failed in clinical development due to disappointing efficacy and unknown toxicity. The peptide-based self-assembly strategy would be promising for utilization of the EPR effect in new drug development, considering previously developed polymer-based nanomaterials such as N-(2-hydroxypropyl)methacrylamide-DOX conjugates (PK1 and PK2) showed poor results in clinical trials [68]. The programs for developing nanomaterials including PK1 and PK2 were have been largely discontinued. Numerous other complicated polymer-based NPs have also failed in clinical trials due to challenges such as high cost of the manufacturing and unwanted toxicity.
In addition to cathepsin B, various tumor-specific enzymes such as matrix metalloproteinase and caspase, have been utilized to improve the EPR effects of nanomedicines [64, 69]. In the case of caspase-3-responsive prodrugs, the induction of apoptosis by external stimuli is initially required to cause the overexpression of caspase-3 in the targeted region of tumor [70]. The induced caspase-3 activates the prodrug, and the released anti-cancer drugs further activate the prodrugs by exerting cytotoxic effects on neighboring cancer cells. Interestingly, this repetitive and sequential process -the induction of caspase-3 and activation of the prodrug- propagated the induction of apoptosis and amplified therapeutic effects [70, 71]. Similar to the mechanism of enzyme-responsive nanomedicine, glutathione disulfide has been utilized for developing reduction-responsive nanomedicine [72, 73].
Onivyde, a recently approved anti-cancer nanomedicine based on irinotecan can be metabolized to form the active metabolite by serine hydrolase or carboxylesterase. Onivyde has successfully performed in clinical use with improved response rates and therapeutic efficacy [74, 75]. In particular, recent findings showed that liposomal irinotecan could completely eliminate the tumors in apoptotic conditions (by radiation therapy) unlike irinotecan itself [76], because it can accumulate in TME and then be metabolized to the active form by tumor-associated macrophages (TAMs). Recent advances further demonstrated that enzyme-triggered supramolecular self-assembly enabled controlled prodrug activation and exhibited increased therapeutic efficacy against cancer cells [77, 78]. This self-assembly property of nanomedicine tends to increase the accumulation of anti-cancer drugs in tumor cells. In conclusion, nanotechnology combined with prodrugs and tumor-related enzymes effectively contributes to the enhanced efficacy and safety of nanomedicine, improving the EPR effect.
Cathepsin B-specific amphiphilic prodrug (FRRG-DOX; Phe-Arg-Arg-Gly-doxorubicin) that can form a stable NP structure in aqueous conditions accumulates in the tumor site by the EPR effect. The NPs can recover their cytotoxicity by cathepsin B (tumor-specific enzyme) at the tumor site after accumulation, and then show therapeutic effect by the intercalation of DOX into DNA. Adapted with permission from [66], copyright 2019 Elsevier.
3. Alteration of TME aided by external sources
Alteration of TME aided by external sources such as laser light, ultrasound and radiation in combination with nanomedicine treatment can improve the EPR effect, overcoming the heterogeneous intensity of the EPR effect. Photodynamic therapy (PDT), sonodynamic therapy (SDT) and radiation therapy (RT) can reconfigure the TME by widening vessel leakiness or destroying physical barriers in TME, which can improve drug accumulation and therapeutic efficacy. These approaches have drawn significant attention, due to the universal use against solid tumors and their excellent safety.
3.1. Combination with PDT
For the EPR effect, blood vessels of the tumor should be leaky, and NPs should effectively pass through these leaky blood vessels and accumulate within the tumor site. However, vessel leakiness is not sufficient in certain tumors, which results in limited extravasation into tumor tissue and suboptimal EPR effect. Unlike subcutaneous tumor model which have a high level of vessel leakiness caused by rapid tumor development, human tumors are known to have insufficient vessel leakiness for high EPR effects. Therefore, technology to enhance vessel leakiness in tumors is essential for successful clinical translation. A photodynamic stimulus can induce permeabilization of vessels in tumor sites, facilitating the extravasation of NPs [79, 80]. After PDT enhances the vessel leakiness in tumor tissue, injected NPs can pass through the leaky blood vessels to accumulate more efficiently within the tumor. In other words, PDT can improve the EPR effect of subsequently administered NPs [67]. PDT is considered a safe and minimally invasive therapeutic procedure. Therefore, nanomedicine treatment combined with PDT attracts increasing attention for successful clinical translation.
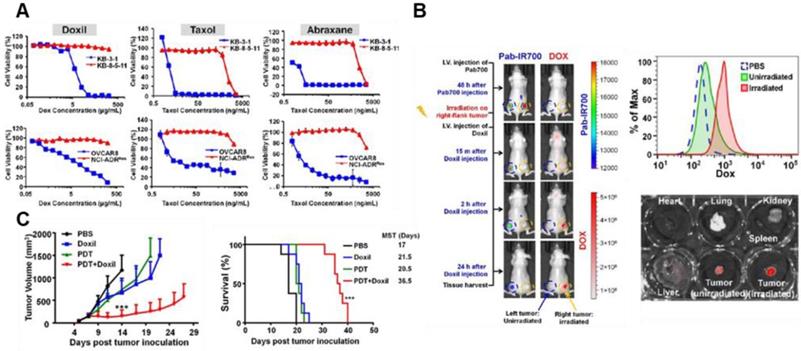
Recent strategies to modulate TME by PDT have been implemented to enhance the anti-tumor effect of nanomedicine and overcome tumor heterogeneity. Various NPs for PDT have showed a synergistic tumor-targeting effect with chemotherapy against malignant cancer [81]. For example, recently developed PDT-utilizing NPs enhanced the anti-cancer effects of FDA-approved cancer nanomedicines by effectively depleting the MDR of cancer cells and increasing their tumor penetration [82]. These results are important because current outcomes of conventional nanomedicines relying solely on the EPR effect have been limited due to unsatisfactory cellular internalization of nanomedicine and reduced drug efflux; all FDA-approved nano-drugs are substrates of multidrug-resistance P-glycoprotein (Pgp) (Figure 5A) [82]. Combined therapy with PDT not only can destroy resistant cancer cells but also enhances the tumor penetration of nanomedicines (Figure 5B). As a result, PDT enhances the efficacy of nanomedicine, which results from reduced interstitial pressure, destroyed biological barriers, and the following increased the EPR effect (Figure 5C). Similarly, synergistic effect resulting from boosted drug release in PDT combined with chemotherapy shows promise as an alternative avenue to achieve the enhanced EPR effect. Disassembly of NPs by PDT at the tumor site boosted cytotoxic drug release, thus activating a cascade of chemotherapeutic effects and then destroying tumor cells in a drug-resistant tumor model [83]. This study implies that PDT can provide a way to overcome critical biological barriers to nanomedicine delivery, which cannot be done with nanomedicine alone. Yan et al. recently developed nano-photosensitizers (isophthalic acid/layered double hydroxide nanohybrids) that can show superior cytotoxic properties with a remarkable IC50 (approximately 0.1 μg/mL) and safety. Its high safety with superior activity may enable clinical translation allied with the stronger EPR effect [84]. The supramolecular photosensitizers take advantage of the NIR laser (808 nm) based markable tissue penetration and exhibit a strong ability to ablate tumors by laser irradiation in vivo. Taking recent research into consideration, PDT-utilizing NPs with the EPR effect would provided effective approaches for clinical translation of cancer nanomedicines in cancer therapy.
Drug resistance of nanomedicines and PDT (A) Cytotoxicity test (72 h incubation) of Doxil, Taxol, and Abraxane in KB-3-1, KB-8-5-11 and OVCAR8 cells (Pgp negative and chemosensitive) shows that therapeutic efficacy can be greatly limited by Pgp-mediated drug resistance. Red color indicates the Pgp overexpressing cells. (B) Pgp-targeted irradiation with PDT enhances the accumulation of Doxil at the tumor site and its tissue penetration. (C) Tumor growth test in xenograft-bearing mice shows that the combination of Doxil and PDT markedly outperforms the two single treatments in showing improved therapeutic efficacy. Data are presented as the mean ± SD (n = 8, ***p < 0.001). Adapted with permission from [82], copyright 2018 Ivyspring International Publisher.
3.2. Combination with SDT
SDT is a emerging non-invasive approach for cancer therapy through activating sonosensitizers by low-energy ultrasound. Sonosensitzers at tumor sites are triggered by ultrasound and then generate reactive oxygen species (ROS) for cancer therapy. SDT has been considered a desirable option for combination with nanomedicine to treat cancers. SDT has sufficient tissue-penetrating depth compared to light in PDT, which is preferable for treating deep-seated tumors and improving the EPR effect of nanomedicine. In addition, SDT can reduce their side effects on normal cells and tissues by its site-specific targeting effect, which can facilitate clinical translation. Over the years, several strategies combining nanomedicine and SDT have been developed in combination with the EPR effect to enhance the therapeutic outcome of anti-cancer therapy. Recent studies show that the EPR effect of sonosensitizers can be improved with a new type of self-assembled nanosonosensitizers. Hangrong Chen et al. developed sonosensitizer-containing NPs that facilitate transferrin receptor-mediated endocytosis for deep tissue penetration [85]. These NPs intercellularly deliver sonosensitizers protoporphyrin IX through transferrin mediation, overcoming the tissue barrier and improving the EPR effect. In addition, high-intensity focused ultrasound (HIFU) can also be used to enhance the EPR effect of nanomedicine, utilizing non-ionizing ultrasonic waves to induce hyperthermia within target tissue [86]. Hyperthermia has been reported to increase the extravasation of NPs into the tumor tissues without toxicity in mice [87]. HIFU has been evaluated in various preclinical studies and has been recognized to be relatively close to clinical translation. In view of the above, there are several preclinical tests of SDT in combination with nanomedicine in progress, addressing delivery issue of the sonosensitizers [88]. Therefore, considering the evidences of therapeutic effects in SDT with the EPR effect, the combination approach with nanomedicine and SDT for cancer treatment would be promising.
3.3. Combination with RT
RT is one of the most commonly used treatments for cancer, either as a monotherapy or in combination with other treatments. RT has formed the mainstream of cancer treatment because it is cost-effective, target-specific and highly effective. Notably, RT is a remarkable therapeutic modality for cancer that is not limited by tissue penetration, destroying tumor cells and the TME. However, although external irradiation can directly kill cancer cells through ROS and energy, RT still has many inevitable shortcomings as single treatment, including dose limitations, severe toxicity and RT resistance [89]. Recently, advances in nanotechnology utilizing the EPR effect have enabled novel strategies for nanomedicine treatment in combination with RT [90]. In fact, RT significantly increases vascular permeability, which enhances the extravasation of subsequently administered nanomedicine from the vessels and its accumulation in tumor sites [91]. A recent study examining the effect of TME-targeted NPs (with RGD) in combination with RT has shown that radiation influences endothelial cells and blood vessels and enhances the therapeutic response to subsequent chemotherapy [92]. Clinically, RT is usually administered at low fractionated doses (1.8-3 Gy), sometimes supplemented with a boost dose at the beginning. It has been reported that low doses of RT are able to induce NP accumulation by destroying immature blood vessels. Therefore, the increased vessel permeability by RT has great potential to improve the delivery of nanomedicine via the EPR even in heterogeneous tumors.
Great advances in nanotechnology have substantially diversified the use of PDT, SDT and RT in combination with nanomedicine. Improvement of the EPR effect with PDT, SDT, and RT with the subsequent administration of nanomedicine for chemotherapy, immunotherapy, or RNA interference therapy has synergistically promoted anti-cancer activity [93]. Because these therapeutic modalities have shown effectiveness, excellent safety and minimal invasiveness, they are considered to have the great potential to enter into the mainstream of cancer therapy. Alternations to the TME by PDT, SDT, and RT would contribute to overcoming the barriers to the delivery of nanomedicine, which would facilitate successful clinical translation. In a different way with these three methods, magnetic NPs can be efficiently accumulated at tumor tissue by improving the EPR effect. Since magnetic NPs have been clinically in use as a magnetic resonance imaging (MRI) contrast agent from the 1990s, magnetic NPs have been extensively investigated for their clinical applications as DDS [94, 95]. Unfortunately, PDT, SDT and RT have been revealed a lack of selectivity of their sensitizers. Therefore, the selective delivery of sensitizing agents into TME using NPs would be optimal option for reducing off-target toxicity and enhancing therapeutic outcome. Overall, these combination strategies incorporating the help of external sources may provide a way to reassess therapeutic agents previously evaluated as suboptimal.
4. Physiological remodeling of TME
One of the major challenges that current nanomedicine has yet to address is how to predict the therapeutic efficacy or delivery efficacy of a nanomedicine without understanding the complicated and heterogeneous TME. Multiple studies have demonstrated that the physiological remodeling of TME substantially improves the delivery of drugs to the tumor site. Current strategies for remodeling TME have focused on biologically changing the structural properties of the tumor and its environment to increase nanomedicine delivery to the target sites [12, 96]. In this section, we discuss promising TME remodeling approaches to improve the EPR effect: the induction of artificial TME and vascular remodeling.
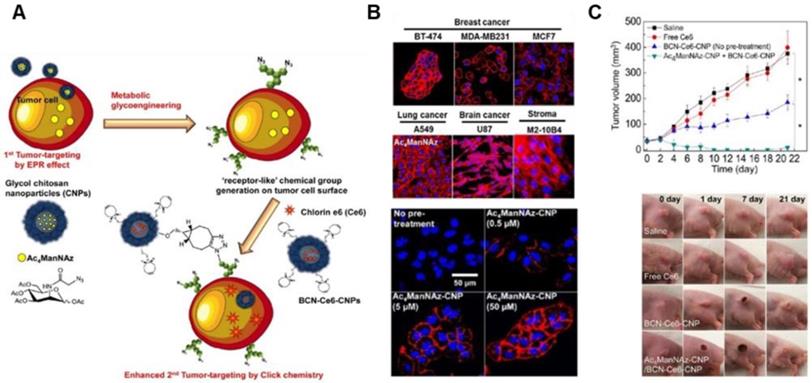
The EPR effect-aided tumor targeting based on metabolic glycoengineering and click chemistry. (A) Schematic illustration of artificial reporter-targeting strategy and mechanism. Adapted with permission from [98], copyright 2014 American Chemical Society. (B) Confocal microscopy images of the generation of azide groups in various Ac4ManNAz-treated cells. Adapted with permission from [99], copyright 2018 Elsevier. (C) Tumor inhibitory effect of chitosan nanoparticle (CNP)-based NPs with Ce6 for PDT in A549 tumor-bearing mice and tumor images. Adapted with permission from [98], copyright 2014 American Chemical Society.
4.1. Induction of artificial TME
The heterogeneity of tumors and the complexity of TME have often presented obstacles even to nanomedicine designed to bind TME-specific molecular markers. Various subpopulations of tumor cells express different kinds and amounts of natural receptors in the TME. To overcome the heterogeneity and complexity of TME, several researchers introduced artificial chemical receptors that are exogenously generated on tumor cells, regardless of phenotypes of tumor cells. In this way, genetically different and heterogeneous tumor cells can be converted into phenotypically uniform cells, leading to a uniform EPR effect for subsequently administered nanomedicine (Figure 6A) [97, 98]. Previous studies have shown that an artificial azide reporter, originating from the metabolic precursor, tetraacetylated N-azidoacetyl-D-mannosamine (Ac4ManNAz) can be presented on the surface of tumor cells by metabolic glycoengineering (Figure 6B) [99, 100]. Subsequently, Chlorin e6 (Ce6)-containing NPs which are decorated with the ligands, bicycle[6.1.0]nonyne to bind to artificial receptors were administered and bound to artificial receptors on tumors through bioorthogonal click reaction between azide groups and the ligands, leading to photodynamic therapy in vivo regardless of tumor types (Figure 6C) [98]. To generate tumor-specific artificial receptors on the tumor cell surface, peptides responsive to tumor-associated enzymes such as cathepsin B or caspase-3/7 were incorporated into metabolic precursor [101]. Peptides responsive to tumor-associated enzymes allow selective induction of artificial receptors on tumor cells. This approach introduced “receptor-like” chemical groups on the surface of tumor cells regardless of tumor types instead of utilizing natural receptors for tumor targeting, demonstrating a way to overcome the heterogeneity of the EPR effect and to improve the EPR effect.
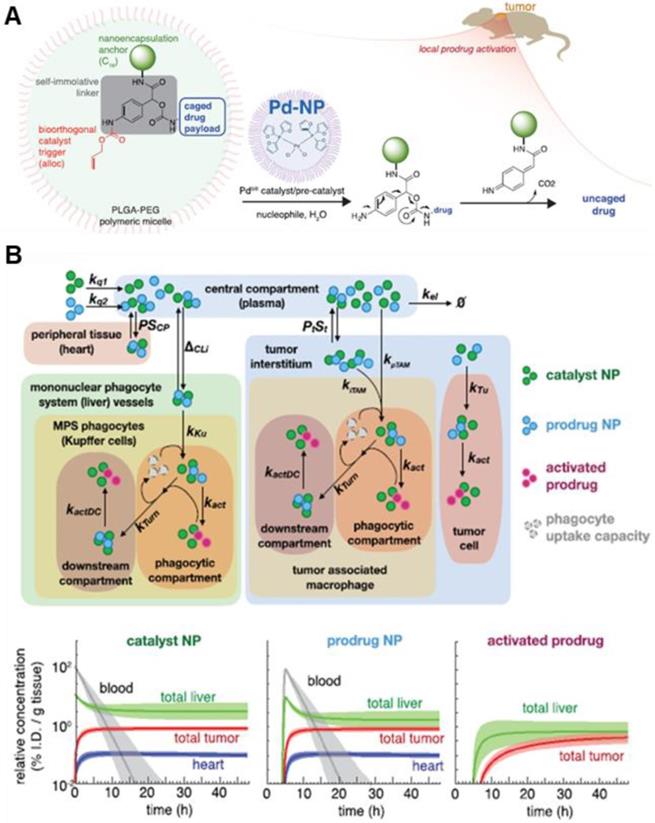
In addition to artificial receptors, artificial ECM was constructed to inhibit tumor invasion and metastasis using laminin-mimic peptide-based NPs [102]. Laminin, high-molecular weight protein of the basal lamina, is one of the most significant components of ECM, influencing cell differentiation and adhesion. Laminin-mimic peptide-based NPs accumulated in the tumor site due to the EPR effect and transformed into ECM around the tumor, which significantly inhibited lung metastasis in melanoma and breast tumor models. This study is somewhat different from the other studies discussed in this review. While other studies sought to enhance drug delivery to tumors by altering ECM, this study sought to inhibit the movement of tumor cells by altering ECM. Recently, Weissleder et al. reported that a palladium catalyst encapsulated in NP (Nano-palladium) demonstrated efficacious catalytic activity in vivo in animal models [78]. Change of in vivo catalytic activity in the tumor sites can be regarded as creating artificial TME for enhanced drug efficacy. Nano-palladium accumulates in solid tumors due to the EPR effect and then activates a DOX prodrug at the tumor site of the tumor model (Figure 7A). Furthermore, a computational multicompartment model of prodrug activation showed the biodistribution of NPs, prodrug, and activated prodrug over 48 h (Figure 7B).
Transforming growth factor beta (TGF-β) has played an important role in the conventional TME remodeling, regulating angiogenesis [103] and inhibiting the expansion of T cells [104]. Remodeling of the TME by inhibiting TGF-β enables NPs to effectively penetrate the targeted tumor tissue. Kano et al. reported that low-doses of TGF-β inhibitor successfully altered the TME including tumor vasculature, which increased the EPR effect with minimal side effects [105]. Additionally, TGF-β inhibition in pericyte-abundant BxPC-3 pancreatic cancer vessels increased the uptake of NPs, logically implying the augmented EPR effect [106, 107]. The role of other TME-related factors, such as fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) is under evaluation in terms of TME-remodeling for anti-cancer therapy.
Schematic illustration of NP with bioorthogonal catalyst trigger (allyloxycarbonyl; alloc), prodrug and Pd-based NP (A) The cleavable protective group (alloc) can be removed by a triggering agent (Pd catalyst) when Pd NPs are delivered to the local tumor site by the EPR effect. (B) The calculated computational multicompartment pharmacokinetic model for the catalyst and prodrug NPs show that biodistribution of NPs and activated prodrug over 48 h. Analysis of the results of simulating the use of a combination of catalyst and prodrug NPs by the EPR effect indicates increased selectivity of drug activation in the tumor. Adapted with permission from [78], copyright 2018 American Chemical Society.
4.2. Vascular remodeling
The delivery of EPR-dependent nanomedicine strongly depends on the characteristic of the tumor vasculature, which directly affects the “permeation” of nanomedicine. Therefore, vascular remodeling using DDS has attracted great attention over the last three decades [11, 108]. In general, highly pro-angiogenic tumors result in low pericyte coverage and loose cell junctions, leading to leaky and disorganized blood vessels in TME [109]. In turn, it reconstructs highly permeable immature vasculature and the elevated interstitial fluid pressure at the tumor site, lead to a high EPR effect [110, 111]. At this present, tumor vessel modulation could be an effective way to influence the intensity of the EPR effect. Approaches to remodel the tumor vasculature using anti-angiogenic agents or external stimuli clearly alter in the function of vessels, affecting permeation and disruption [112]. In addition, there are approaches that enlarge the endothelial pores which serve as the gateway for nanomedicine to extravasate into TME, by using vasodilators and vasoactive cytokines, such as nitric oxide (NO), nitroglycerine, tumor necrosis factor-alpha (TNF-α), angiotensin-Ⅱ, etc. The effect of the leaky vasculature is discussed in Section 3.1. Xu et al., for instance, reported that NO-releasing NPs combining a photosensitizer (IR780) and chemotherapeutic drug (PTX) significantly suppressed tumor growth by boosting tumor vascular permeability [113].
There are many approaches to inhibit new blood vessel formation, not only reducing the supply of oxygen and nutrients to tumor, but also leading to the regression of established tumors [114]. The normalization of tumor blood vessels in TME can be achieved by the administration of anti-vascular endothelial growth factor (VEGF) antibodies or VEGF receptor inhibitors [115, 116]. It has also been reported that vascular stabilization and maturation are strongly influenced by tyrosine kinase with immunoglobulin-like and EGF-like domains 2 (Tie2) [117]. Among FDA-approved therapeutic antibodies against cancer, trastuzumab (marketed as Herceptin®) could improve vascular perfusion for the retention of nanomedicines at the tumor site [118, 119]. Among these anti-angiogenic therapies, VEGF inhibition has taken center stage due to the significance of the correlation between VEGF and angiogenesis in cancer. Anti-VEGF therapy induces synergistic anti-tumor effects through normalization of tumor vessels [120, 121]. VEGF inhibition using siRNA improves the efficacy of concurrent chemotherapy, which may be attributed to enhanced permeation of chemotherapeutic drug-containing NPs [122]. On the other hand, vascular-disrupting agents (VDAs) have been used to induce the rapid collapse of tumor vasculature, resulting in vascular remodeling [123]. Additionally, appropriate levels of angiogenesis i.e., a certain density and even distribution of tumor blood vessels, practically increase total blood volume and improve perfusion, resulting in enhanced drug accumulation in tumors [124, 125]. This alternative approach which is distinct from vascular normalization is called vascular promotion, improves the efficacy of co-administered therapeutics, such as chemotherapeutic drug-containing NPs [126] and anti-PD-1 antibodies [127]. This physical change in the TME helps to generate microvascular networks and normal vessels in ECM, contributing to the improvement of the EPR effect [128]. Another interesting strategy for improving vascular remodeling is to use anti-hypertensive agents such as losartan, an inhibitor of angiotensin. It has been reported that targeting angiotensin signaling with an angiotensin inhibitor can diminish the ECM, especially collagen and hyaluronan [129, 130]. Recently, Zhao et al. reported that losartan reduced the physical forces actively exerted by tumor and stromal cells to compress tumor blood vessels in ovarian carcinoma xenograft models. This reduction leads to enhanced delivery and efficacy of the chemotherapeutic drug, PTX via improving vascular perfusion [131]. Losartan combined with chemotherapeutic drugs is undergoing clinical trial (National Clinical Trial [NCT] identifier; NCT01821729) for pancreatic tumor treatment. Overall, these diverse approaches to modifying the tumor vasculature mainly affect the permeation of nanomedicine, improving the EPR effect.
The induction of artificial TME, including artificial receptors and ECM, can provide a promising opportunity for improved therapeutic outcomes by neutralizing the inherent heterogeneity of tumor and adding extrinsic homogeneity to the tumor. These approaches can be further expanded to provide a tool for personalized medicine to match the specific need of individual tumors. Although this review does not include immunotherapy, nanomedicine can be combined with immunotherapy agents to overcome the drug resistance and boost the immune response by remodeling the immune cell composition in the TME. For example, remodeling of tumor stroma composed of cancer-associated fibroblasts (CAFs), altering anti-tumor immune response [132] and deleting immune cells to protect tumors [133], has recently come into the spotlight. DDS needs a strategy for tearing down the 'walls' of CAFs to improve the EPR effect and immunotherapy. The CAFs affect the functional polarization of TAMs, which can phagocytize NPs and are thus indirectly able to enhance drug delivery and accumulation [134]. Consequently, the EPR effect is improved by increased macrophage infiltration into the TME [135]. TAMs are one of the most abundant immune cell populations in the TME and are critical modulators of the TME that directly affect vascularization and ECM remodeling [136]. TAMs directly affect tumor vascularization by secreting pro-antigenic factors and directly affect ECM remodeling by releasing ECM-degrading enzymes or by stimulating collagen secretion. These factors make TAMs an attractive target for increasing the EPR effect in cancer treatment. For example, cyclic tumor homing peptide iRGD (CCRGDKGPDC)-based NPs with a macrophage-specific sequence (AAN) could inhibit tumor growth and modulate TME with depletion of TAM [137]. In this study, a significant improvement in anti-tumor efficacy and NPs accumulation was achieved by interaction with tumor vascular endothelial cells, indicating the increased EPR effect. The macrophages could be polarized into TAMs, pro-tumorigenic M2 macrophages or anti-tumorigenic M1 macrophages depending on signals in the surrounding environment [138]. Selective delivery into TAMs or M1 macrophages is necessary to remodel TME to favor cancer treatment. Zhu et al. created PEGylated cowpea mosaic virus particles that could be internalized by TAMs but not M1 macrophages. They suggested that these NPs can be loaded with cytotoxic agents that target the population of TAMs only, causing the population of M1 to rise as the population of TAMs decreases. In addition, as phagocytic inflammatory cells, macrophages are commonly known to be able to phagocytose particles, thereby inducing off-target effects of nanomedicines [139]. Davis et al. proved that the cellular uptake of TAMs was approximately 3.5-fold greater than that of LKB498 melanoma cancer cells by radiolabeling [140]. TAMs could reduce the uptake of NPs by cancer cells in the TME and TAM depletion could thus decrease the off-target uptake of NPs. Therefore, TME modulation by TAM depletion is able to indirectly enhance the EPR effect in vivo. Taken together, it increasingly becomes evident that modern immunotherapy can greatly benefit from the expansion of our understanding of nanotechnology and DDS.
5. Conclusions and perspectives
Passive targeting strategies based on the EPR effect have shown great therapeutic potential in various preclinical animal models. However, the therapeutic outcome of passively targeted nanomedicine in clinical practice is heterogeneous mainly due to the inherent heterogeneity of the EPR effect. Additionally, the low delivery efficiency of NPs to a solid tumor (approximately 1-5%) indicates that the EPR effect itself may have fundamental limitations for clinical application [141]. For the translation of NPs from animal studies to the clinic, various factors such as tumor size, type, and location must be considered carefully [10, 142]. In fact, the EPR effect was reported to be maximized in tumors that contain low intratumoral ECM and large amounts of angiogenic blood vessels. This hurdle causes to the limited number of nanomedicines in the current markets since Doxil® (liposomal DOX) emerged as the first nanomedicine in 1995. Over the last two decades, several other nano-drugs including Abraxane (NP with albumin-bound PTX) and Onivyde (liposomal irinotecan) successfully entered the market, showing remarkable therapeutic efficacy in patients. To increase the clinical use of nanomedicine, the low clinical efficacy of the EPR effect also needs to be overcome by novel approaches. In this point of view, recent clinical studies show that novel NPs based strategies enable the design of tumor-specific nanocarriers that recognize biological targets in TME. For example, anti-EGFR-immunoliposomes loaded with DOX have shown high efficacy and low toxicity in phase I and II clinical trials (NCT02833766) for the patients with advanced triple-negative and EGFR-positive breast cancer. Also, ThermoDox which is a heat-activated liposome of DOX, coupled with radiofrequency-induced heating, is in phase III clinical trials for the treatment of hepatocellular carcinoma (NCT02112656) [143]. Taken together, these approaches have shown promises for clinical translation of nanomedicine, overcoming the limitations of the EPR effect alone to treat solid tumors such as pancreatic and breast cancer.
In this review, we focused on the current attempts at overcoming the limitations of traditional EPR-dependent nanomedicine by combining with supplementary strategies, such as additional molecular targeting, physical alteration, or physiological remodeling of the TME. The diverse attempts discussed in this review present both limitations and promise for clinical use. To overcome these limitations, further studies are necessary. NPs that are designed to bind TME-specific molecular markers can become more impactful with studies that identify new crucial targets in tumor ECM. In turn, the new findings will lead to innovative nanomedicine platforms that are suitable for the new targets. Additionally, as our understanding of the complex TME and heterogeneity of tumors expands, nanomedicine with multiple complementary targets may prove more beneficial for precise localization of drugs in the body and therapeutic outcomes in the clinic. If the tumor site is externally accessible, another favorable approach is local delivery combined with PDT and SDT to improve the EPR effect, especially in tumors with a low initial EPR effect. Furthermore, inducing the expression of an artificial receptor on the surface of heterogeneous tumor cells can provide an alternative opportunity for the better therapeutic outcome by improving the EPR effect and by adding extrinsic homogeneity to the tumor. The induction of artificial receptors can further be personalized to match the specific needs of individual tumors.
We propose that additional strategies applied in combination with the EPR effect should address the specific characteristics of the TME in various cancers. These additional approaches might greatly advance to the current treatment options for solid tumors (such as colon, breast and pancreatic cancer) with low EPR, overcoming the current limitation of its clinical application of nanomedicine. In this regard, assessing the EPR effect in individuals is crucial for improved therapeutic effects. Clinically available technology for imaging the EPR effect in patients, such as computed tomography, MRI could provide clinicians with valuable information for medication regimens and treatment planning, thus paving the way for personalized nanomedicine [144, 145]. Individuals with tumors that exhibit high EPR would be treated with EPR-dependent nanomedicine, while combination treatment in addition to traditional nanomedicine would be applied to the individuals with tumors that produce a low EPR effect. Unfortunately, the current status of EPR imaging is at the developmental stage; thus far, few studies have assessed or clinically analyzed the EPR effect in patients. However, the rapid ongoing developments in nanotechnology, the call for more personalized treatments, and the concurrent expansion of the additional strategies available in conjunction with traditional nanomedicine discussed in this review will doubtlessly raise the number of clinically approved nanomedicines and extend their benefits to more patients in need.
Acknowledgements
This work was supported by grants from the National Research Foundation (NRF-2018R1A6A3A01012195) of Republic of Korea, KU-KIST School Project (Graduate School of Converging Science and Technology, Korea University) and the Intramural Research Program (2E29370) of KIST.
Abbreviations
Ac4ManNAz: tetraacetylated N-azidoacetyl-D-mannosamine; CAFs: cancer-associated fibroblasts; Ce6: Chlorin e6; DDS: drug delivery systems; DOX: doxorubicin; EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; EPI: epirubicin; FDA: Food and Drug Administration; HA: hyaluronic acid; HIF-1α: hypoxia-inducible factor-1α; HIFU: high-intensity focused ultrasound; LIFU: low-intensity focused ultrasound; MDR: multi-drug resistance; MRI: magnetic resonance imaging; NIR: near-infrared; PDT: photodynamic therapy; PEG: polyethylene glycol; PEGPH20: PEGylated recombinant human hyaluronidase; PEI: polyethyleneimine; Pgp: P-glycoprotein; PTX: paclitaxel; RGD: Arg-Gly-Asp; ROS: reactive oxygen species; RT: radiation therapy; SDT: sonodynamic therapy; siRNA: small interfering RNA; TAMs: tumor-associated macrophages; TGF-β: Transforming growth factor beta; TNF-α: tumor necrosis factor-alpha; VDAs: vascular-disrupting agents; VEGF: vascular endothelial growth factor.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hadjidemetriou M, Kostarelos K. Nanomedicine: Evolution of the nanoparticle corona. Nat Nanotechnol. 2017;12:288-90
2. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS. et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71
3. Li Z, Tan SR, Li S, Shen Q, Wang KH. Cancer drug delivery in the nano era: An overview and perspectives. Oncol Rep. 2017;38:611-24
4. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271-84
5. Yu XJ, Trase I, Ren MQ, Duval K, Guo X, Chen Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J Nanomater. 2016
6. Clemons TD, Singh R, Sorolla A, Chaudhari N, Hubbard A, Iyer KS. Distinction Between Active and Passive Targeting of Nanoparticles Dictate Their Overall Therapeutic Efficacy. Langmuir. 2018;34:15343-9
7. Bernier-Latmani J, Petrova TV. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol. 2017;14:510-26
8. Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387-92
9. Ventola CL. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T. 2017;42:742-55
10. Park K. Facing the Truth about Nanotechnology in Drug Delivery. ACS Nano. 2013;7:7442-7
11. Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the tumor vasculature. Semin Thromb Hemost. 2010;36:321-31
12. Zhang B, Hu Y, Pang Z. Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery. Front Pharmacol. 2017;8:952
13. Ojha T, Pathak V, Shi Y, Hennink WE, Moonen CTW, Storm G. et al. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv Drug Deliv Rev. 2017;119:44-60
14. Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12:381-94
15. Alizadeh AA, Aranda V, Bardelli A, Blanpain C, Bock C, Borowski C. et al. Toward understanding and exploiting tumor heterogeneity. Nat Med. 2015;21:846-53
16. Marjanovic ND, Weinberg RA, Chaffer CL. Cell plasticity and heterogeneity in cancer. Clin Chem. 2013;59:168-79
17. Hiratsuka S. Vasculogenensis, angiogenesis and special features of tumor blood vessels. Front Biosci (Landmark Ed). 2011;16:1413-27
18. Tan YF, Lao LL, Xiong GM, Venkatraman S. Controlled-release nanotherapeutics: State of translation. J Control Release. 2018;284:39-48
19. Tong X, Wang ZT, Sun XL, Song JB, Jacobson O, Niu G. et al. Size Dependent Kinetics of Gold Nanorods in EPR Mediated Tumor Delivery. Theranostics. 2016;6:2039-51
20. Liu DX, Auguste DT. Cancer targeted therapeutics: From molecules to drug delivery vehicles. J Control Release. 2015;219:632-43
21. Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F. et al. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17-38
22. Zhu YQ, Feijen J, Zhong ZY. Dual-targeted nanomedicines for enhanced tumor treatment. Nano Today. 2018;18:65-85
23. Overchuk M, Zheng G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials. 2018;156:217-37
24. Florence AT. "Targeting" nanoparticles: The constraints of physical laws and physical barriers. J Control Release. 2012;164:115-24
25. Torrice M. Does Nanomedicine Have a Delivery Problem? ACS Cent Sci. 2016;2:434-7
26. Uthaman S, Huh KM, Park IK. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater Res. 2018;22:22
27. Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J Control Release. 2018;277:1-13
28. Anchordoquy TJ, Barenholz Y, Boraschi D, Chorny M, Decuzzi P, Dobrovolskaia MA. et al. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS Nano. 2017;11:12-8
29. Chen HM, Zhang WZ, Zhu GZ, Xie J, Chen XY. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017:2
30. Raave R, van Kuppevelt TH, Daamen WF. Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J Control Release. 2018;274:1-8
31. Chen HJ, Niu G, Wu H, Chen XY. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics. 2016;6:78-92
32. Fu S, Xu XD, Ma Y, Zhang SB, Zhang SF. RGD peptide-based non-viral gene delivery vectors targeting integrin αvβ3 for cancer therapy. J Drug Target. 2019;27:1-11
33. Pasqualini R, Koivunen E, Ruoslahti E. alpha v Integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15:542-6
34. Wang Y, Liu Z, Li T, Chen L, Lyu J, Li C. et al. Enhanced Therapeutic Effect of RGD-Modified Polymeric Micelles Loaded With Low-Dose Methotrexate and Nimesulide on Rheumatoid Arthritis. Theranostics. 2019;9:708-20
35. Fan Z, Chang Y, Cui C, Sun L, Wang DH, Pan Z. et al. Near infrared fluorescent peptide nanoparticles for enhancing esophageal cancer therapeutic efficacy. Nat Commun. 2018;9:2605
36. Wang XY, Wang H, Jiang K, Zhang YY, Zhan CY, Ying M. et al. Liposomes with cyclic RGD peptide motif triggers acute immune response in mice. J Control Release. 2019;293:201-14
37. Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3-20
38. Ryu JH, Shin M, Kim SA, Lee S, Kim H, Koo H. et al. In vivo fluorescence imaging for cancer diagnosis using receptor-targeted epidermal growth factor-based nanoprobe. Biomaterials. 2013;34:9149-59
39. Zalba S, Contreras AM, Merino M, Navarro I, de Ilarduya CT, Troconiz IF. et al. EGF-liposomes promote efficient EGFR targeting in xenograft colocarcinoma model. Nanomedicine (Lond). 2016;11:465-77
40. Choi KY, Han HS, Lee ES, Shin JM, Almquist BD, Lee DS. et al. Hyaluronic Acid-Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv Mater. 2019. 1803 549
41. Liu R, Xiao W, Hu C, Xie R, Gao H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Control Release. 2018;278:127-39
42. Lv YQ, Xu CR, Zhao XM, Lin CS, Yang X, Xin XF. et al. Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano. 2018;12:1519-36
43. Chen J, Ouyang J, Chen QJ, Deng C, Meng FH, Zhang J. et al. EGFR and CD44 Dual-Targeted Multifunctional Hyaluronic Acid Nanogels Boost Protein Delivery to Ovarian and Breast Cancers In vitro and In vivo. ACS Appl Mater Interfaces. 2017;9:24140-7
44. Liang YY, Peng JH, Li N, Yu-Wai-Man C, Wang Q, Xu YH. et al. Smart nanoparticles assembled by endogenous molecules for siRNA delivery and cancer therapy via CD44 and EGFR dual-targeting. Nanomedicine. 2019;15:208-17
45. Thompson CB, Shepard HM, O'Connor PM, Kadhim S, Jiang P, Osgood RJ. et al. Enzymatic Depletion of Tumor Hyaluronan Induces Antitumor Responses in Preclinical Animal Models. Mol Cancer Ther. 2010;9:3052-64
46. Infante JR, Korn RL, Rosen LS, LoRusso P, Dychter SS, Zhu J. et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br J Cancer. 2018:118
47. Doherty GJ, Tempero M, Corrie PG. HALO-109-301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018;14:13-22
48. Zeng W, Liu P, Pan W, Singh SR, Wei Y. Hypoxia and hypoxia inducible factors in tumor metabolism. Cancer Lett. 2015;356:263-7
49. Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S. et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35:1735-43
50. Aldea M, Florian IA, Kacso G, Craciun L, Boca S, Soritau O. et al. Nanoparticles for Targeting Intratumoral Hypoxia: Exploiting a Potential Weakness of Glioblastoma. Pharm Res. 2016;33:2059-77
51. Blanco VM, Chu Z, Vallabhapurapu SD, Sulaiman MK, Kendler A, Rixe O. et al. Phosphatidylserine-selective targeting and anticancer effects of SapC-DOPS nanovesicles on brain tumors. Oncotarget. 2014;5:7105-18
52. Masoud GN, Li W. HIF-1 alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378-89
53. Jain S, Pathak K, Vaidya A. Molecular therapy using siRNA: Recent trends and advances of multi target inhibition of cancer growth. Int J Biol Macromol. 2018;116:880-92
54. van den Brand D, Mertens V, Massuger L, Brock R. siRNA in ovarian cancer - Delivery strategies and targets for therapy. J Control Release. 2018;283:45-58
55. Perche F, Biswas S, Patel NR, Torchilin VP. Hypoxia-Responsive Copolymer for siRNA Delivery. Methods Mol Biol. 2016;1372:139-62
56. Wang J, Meng F, Kim BK, Ke X, Yeo Y. In-vitro and in-vivo difference in gene delivery by lithocholic acid-polyethyleneimine conjugate. Biomaterials. 2019;217:119296
57. Thambi T, You DG, Han HS, Deepagan VG, Jeon SM, Suh YD. et al. Bioreducible carboxymethyl dextran nanoparticles for tumor-targeted drug delivery. Adv Healthc Mater. 2014;3:1829-38
58. Qian C, Yu J, Chen Y, Hu Q, Xiao X, Sun W. et al. Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy. Adv Mater. 2016;28:3313-20
59. Estrella V, Chen TA, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A. et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013;73:1524-35
60. Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T. et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013:13
61. Han SS, Li ZY, Zhu JY, Han K, Zeng ZY, Hong W. et al. Dual-pH Sensitive Charge-Reversal Polypeptide Micelles for Tumor-Triggered Targeting Uptake and Nuclear Drug Delivery. Small. 2015;11:2543-54
62. Liu JJ, Liang HN, Li MH, Luo Z, Zhang JX, Guo XM. et al. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials. 2018;157:107-24
63. Yao MN, Ma XT, Zhang X, Shi LQ, Liu TY, Liang XL. et al. Lectin-Mediated pH-Sensitive Doxorubicin Prodrug for Pre-Targeted Chemotherapy of Colorectal Cancer with Enhanced Efficacy and Reduced Side Effects. Theranostics. 2019;9:747-60
64. Abumanhal-Masarweh H, Koren L, Zinger A, Yaari Z, Krinsky N, Kaneti G. et al. Sodium bicarbonate nanoparticles modulate the tumor pH and enhance the cellular uptake of doxorubicin. J Control Release. 2019;296:1-13
65. Rautio J, Meanwell NA, Di L, Hageman MJ. The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov. 2018;17:559-87
66. Shim MK, Park J, Yoon HY, Lee S, Um W, Kim JH. et al. Carrier-free nanoparticles of cathepsin B-cleavable peptide-conjugated doxorubicin prodrug for cancer targeting therapy. J Control Release. 2019;294:376-89
67. Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev. 2017;108:25-38
68. Ruan H, Hao S, Young P, Zhang H. Targeting Cathepsin B for Cancer Therapies. Horiz Cancer Res. 2015;56:23-40
69. Wang ZH, Wang YH, Jia XQ, Han QJ, Qian YX, Li Q. et al. MMP-2-Controlled Transforming Micelles for Heterogeneic Targeting and Programmable Cancer Therapy. Theranostics. 2019;9:1728-40
70. Lee BS, Cho YW, Kim GC, Lee DH, Kim CJ, Kil HS. et al. Induced phenotype targeted therapy: radiation-induced apoptosis-targeted chemotherapy. J Natl Cancer Inst. 2015:107
71. Ul Khaliq N, Sandra FC, Park DY, Lee JY, Oh KS, Kim D. et al. Doxorubicin/heparin composite nanoparticles for caspase-activated prodrug chemotherapy. Biomaterials. 2016;101:131-42
72. Sun BJ, Luo C, Yu H, Zhang XB, Chen Q, Yang WQ. et al. Disulfide Bond-Driven Oxidation- and Reduction-Responsive Prodrug Nanoassemblies for Cancer Therapy. Nano Lett. 2018;18:3643-50
73. Luo C, Sun B, Wang C, Zhang X, Chen Y, Chen Q. et al. Self-facilitated ROS-responsive nanoassembly of heterotypic dimer for synergistic chemo-photodynamic therapy. J Control Release. 2019;302:79-89
74. Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res. 2016;33:2373-87
75. Lammers T, Kiessling F, Ashford M, Hennink W, Crommelin D, Storm G. Cancer nanomedicine: is targeting our target?. Nat Rev Mater. 2016:1
76. Miller MA, Chandra R, Cuccarese MF, Pfirschke C, Engblom C, Stapleton S. et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci Transl Med. 2017:9
77. Yao QX, Lin F, Fan XY, Wang YP, Liu Y, Liu ZF. et al. Synergistic enzymatic and bioorthogonal reactions for selective prodrug activation in living systems. Nat Commun. 2018:9
78. Miller MA, Mikula H, Luthria G, Li R, Kronister S, Prytyskach M. et al. Modular Nanoparticulate Prodrug Design Enables Efficient Treatment of Solid Tumors Using Bioorthogonal Activation. ACS Nano. 2018;12:12814-26
79. Zhen Z, Tang W, Chuang YJ, Todd T, Zhang W, Lin X. et al. Tumor vasculature targeted photodynamic therapy for enhanced delivery of nanoparticles. ACS Nano. 2014;8:6004-13
80. Chen B, Pogue BW, Luna JM, Hardman RL, Hoopes PJ, Hasan T. Tumor vascular permeabilization by vascular-targeting photosensitization: Effects, mechanism, and therapeutic implications. Clin Cancer Res. 2006;12:917-23
81. Li C, Yang XQ, Zhang MZ, Song YY, Cheng K, An J. et al. In vivo Imaging-Guided Nanoplatform for Tumor Targeting Delivery and Combined Chemo-, Gene- and Photothermal Therapy. Theranostics. 2018;8:5662-75
82. Mao CQ, Li F, Zhao Y, Debinski W, Ming X. P-glycoprotein-targeted photodynamic therapy boosts cancer nanomedicine by priming tumor microenvironment. Theranostics. 2018;8:6274-90
83. Sun CY, Cao ZY, Zhang XJ, Sun R, Yu CS, Yang XZ. Cascade-amplifying synergistic effects of chemo-photodynamic therapy using ROS-responsive polymeric nanocarriers. Theranostics. 2018;8:2939-53
84. Gao R, Mei X, Yan D, Liang R, Wei M. Nano-photosensitizer based on layered double hydroxide and isophthalic acid for singlet oxygenation and photodynamic therapy. Nat Commun. 2018;9:2798
85. Zhang Q, Wang N, Ma M, Luo Y, Chen H. Transferrin Receptor-Mediated Sequential Intercellular Nanoparticles Relay for Tumor Deep Penetration and Sonodynamic Therapy. Adv Ther (Weinh). 2019. 1800 152
86. Nakamura H, Fang J, Maeda H. Development of next-generation macromolecular drugs based on the EPR effect: challenges and pitfalls. Expert Opin Drug Deliv. 2015;12:53-64
87. Buckway B, Frazier N, Gormley AJ, Ray A, Ghandehari H. Gold nanorod-mediated hyperthermia enhances the efficacy of HPMA copolymer-Y-90 conjugates in treatment of prostate tumors. Nucl Med Biol. 2014;41:282-9
88. Kwon S, Ko H, You DG, Kataoka K, Park JH. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Accounts Chem Res. 2019;52:1771-82
89. Zhu S, Gu Z, Zhao Y. Harnessing Tumor Microenvironment for Nanoparticle-Mediated Radiotherapy. Adv Ther (Weinh). 2018;1:1800050
90. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409-25
91. Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S. et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016;13:627-42
92. Ashton JR, Castle KD, Qi Y, Kirsch DG, West JL, Badea CT. Dual-Energy CT Imaging of Tumor Liposome Delivery After Gold Nanoparticle-Augmented Radiation Therapy. Theranostics. 2018;8:1782-97
93. Min YZ, Roche KC, Tian SM, Eblan MJ, McKinnon KP, Caster JM. et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877
94. Kang T, Li FY, Baik S, Shao W, Ling DS, Hyeon T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials. 2017;136:98-114
95. Gobbo OL, Sjaastad K, Radomski MW, Volkov Y, Prina-Mello A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics. 2015;5:1249-63
96. Dai YL, Xu C, Sun XL, Chen XY. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46:3830-52
97. Koo H, Lee S, Na JH, Kim SH, Hahn SK, Choi K. et al. Bioorthogonal Copper-Free Click Chemistry In vivo for Tumor-Targeted Delivery of Nanoparticles. Angew Chem Int Ed Engl. 2012;51:11836-40
98. Lee S, Koo H, Na JH, Han SJ, Min HS, Lee SJ. et al. Chemical tumor-targeting of nanoparticles based on metabolic glycoengineering and click chemistry. ACS Nano. 2014;8:2048-63
99. Yoon HY, Shin ML, Shim MK, Lee S, Na JH, Koo H. et al. Artificial Chemical Reporter Targeting Strategy Using Bioorthogonal Click Reaction for Improving Active-Targeting Efficiency of Tumor. Mol Pharm. 2017;14:1558-70
100. Lee S, Jung S, Koo H, Na JH, Yoon HY, Shim MK. et al. Nano-sized metabolic precursors for heterogeneous tumor-targeting strategy using bioorthogonal click chemistry in vivo. Biomaterials. 2017;148:1-15
101. Shim MK, Yoon HY, Ryu JH, Koo H, Lee S, Park JH. et al. Cathepsin B-Specific Metabolic Precursor for In vivo Tumor-Specific Fluorescence Imaging. Angew Chem Int Ed Engl. 2016;55:14698-703
102. Hu XX, He PP, Qi GB, Gao YJ, Lin YX, Yang C. et al. Transformable Nanomaterials as an Artificial Extracellular Matrix for Inhibiting Tumor Invasion and Metastasis. ACS Nano. 2017;11:4086-96
103. Penafuerte C, Bautista-Lopez N, Bouchentouf M, Birman E, Forner K, Galipeau J. Novel TGF-beta antagonist inhibits tumor growth and angiogenesis by inducing IL-2 receptor-driven STAT1 activation. J Immunol. 2011;186:6933-44
104. Dahmani A, Delisle JS. TGF-beta in T Cell Biology: Implications for Cancer Immunotherapy. Cancers (Basel). 2018:10
105. Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M. et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A. 2007;104:3460-5
106. Kamei R, Tanaka HY, Kawano T, Morii C, Tanaka S, Nishihara H. et al. Regulation of endothelial Fas expression as a mechanism of promotion of vascular integrity by mural cells in tumors. Cancer Sci. 2017;108:1080-8
107. Tanaka HY, Kano MR. Stromal barriers to nanomedicine penetration in the pancreatic tumor microenvironment. Cancer Sci. 2018;109:2085-92
108. Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T. et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15:1219-23
109. Ferretti S, Allegrini PR, Becquet MM, McSheehy PM. Tumor interstitial fluid pressure as an early-response marker for anticancer therapeutics. Neoplasia. 2009;11:874-81
110. Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423-35
111. Schaaf MB, Garg AD, Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018;9:115
112. Ojha T, Pathak V, Shi Y, Hennink WE, Moonen CTW, Storm G. et al. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv Drug Deliv Rev. 2017;119:44-60
113. Xu Y, Ren H, Liu J, Wang Y, Meng Z, He Z. et al. A switchable NO-releasing nanomedicine for enhanced cancer therapy and inhibition of metastasis. Nanoscale. 2019;11:5474-88
114. Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW. et al. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci U S A. 2003;100:7785-90
115. Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123:3190-200
116. Wu JB, Tang YL, Liang XH. Targeting VEGF pathway to normalize the vasculature: an emerging insight in cancer therapy. Oncotargets Ther. 2018;11:6901-9
117. Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S. et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat Commun. 2017;8:16106
118. Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumor biology - Herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279-80
119. Sorace AG, Quarles CC, Whisenant JG, Hanker AB, McIntyre JO, Sanchez VM. et al. Trastuzumab improves tumor perfusion and vascular delivery of cytotoxic therapy in a murine model of HER2+breast cancer: preliminary results. Breast Cancer Res Treat. 2016;155:273-84
120. Lee SJ, Yook S, Yhee JY, Yoon HY, Kim MG, Ku SH. et al. Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J Control Release. 2015;220:631-41
121. Abdalla AME, Xiao L, Ullah MW, Yu M, Ouyang CX, Yang G. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics. 2018;8:533
122. Kwak G, Jo SD, Kim D, Kim H, Kim MG, Kim K. et al. Synergistic antitumor effects of combination treatment with metronomic doxorubicin and VEGF-targeting RNAi nanoparticles. J Control Release. 2017;267:203-13
123. Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10:415-27
124. Bridges E, Harris AL. Vascular-promoting therapy reduced tumor growth and progression by improving chemotherapy efficacy. Cancer Cell. 2015;27:7-9
125. Huang D, Lan H, Liu F, Wang S, Chen X, Jin K. et al. Anti-angiogenesis or pro-angiogenesis for cancer treatment: focus on drug distribution. Int J Clin Exp Med. 2015;8:8369-76
126. Wong PP, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MRL, Scudamore CL. et al. Dual-Action Combination Therapy Enhances Angiogenesis while Reducing Tumor Growth and Spread. Cancer Cell. 2015;27:123-37
127. Eino D, Tsukada Y, Naito H, Kanemura Y, Iba T, Wakabayashi T. et al. LPA4-Mediated Vascular Network Formation Increases the Efficacy of Anti-PD-1 Therapy against Brain Tumors. Cancer Res. 2018;78:6607-20
128. Crosby CO, Zoldan J. Mimicking the physical cues of the ECM in angiogenic biomaterials. Regen Biomater. 2019;6:61-73
129. Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909-14
130. Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV. et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516
131. Zhao Y, Cao J, Melamed A, Worley M, Gockley A, Jones D. et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2019;116:2210-9
132. Ziani L, Chouaib S, Thiery J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front Immunol. 2018;9:414
133. Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T Cells to protect tumour cells. Nat Commun. 2018;9:948
134. Cuccarese MF, Dubach JM, Pfirschke C, Engblom C, Garris C, Miller MA. et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat Commun. 2017;8:14293
135. Zhao Y, Zhang CR, Gao LQ, Yu XH, Lai JH, Lu DH. et al. Chemotherapy-Induced Macrophage Infiltration into Tumors Enhances Nanographene-Based Photodynamic Therapy. Cancer Res. 2017;77:6021-32
136. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49-61
137. Song X, Wan ZY, Chen TJ, Fu Y, Jiang KJ, Yi XL. et al. Development of a multi-target peptide for potentiating chemotherapy by modulating tumor microenvironment. Biomaterials. 2016;108:44-56
138. Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1-9
139. Musetti S, Huang L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. ACS Nano. 2018;12:11740-55
140. Roode LE, Brighton H, Bo T, Perry JL, Parrott MC, Kersey F. et al. Subtumoral analysis of PRINT nanoparticle distribution reveals targeting variation based on cellular and particle properties. Nanomedicine. 2016;12:1053-62
141. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF. et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014
142. Park K. Transcending nanomedicine to the next level: Are we there yet? J Control Release. 2019;298:213
143. Tak WY, Lin SM, Wang Y, Zheng J, Vecchione A, Park SY. et al. Phase III HEAT Study Adding Lyso-Thermosensitive Liposomal Doxorubicin to Radiofrequency Ablation in Patients with Unresectable Hepatocellular Carcinoma Lesions. Clin Cancer Res. 2018;24:73-83
144. Xu H, Ohulchanskyy TY, Yakovliev A, Zinyuk R, Song J, Liu L. et al. Nanoliposomes Co-Encapsulating CT Imaging Contrast Agent and Photosensitizer for Enhanced, Imaging Guided Photodynamic Therapy of Cancer. Theranostics. 2019;9:1323-35
145. Zhang QH, Chen JW, Shen J, Chen SX, Liang KC, Wang H. et al. Inlaying Radiosensitizer onto the Polypeptide Shell of Drug-Loaded Ferritin for Imaging and Combinational Chemo-Radiotherapy. Theranostics. 2019;9:2779-90
146. Shim MK, Yoon HY, Lee S, Jo MK, Park J, Kim JH. et al. Caspase-3/-7-Specific Metabolic Precursor for Bioorthogonal Tracking of Tumor Apoptosis. Sci Rep. 2017;7:16635
147. Lee IJ, Lee M, Kim SJ, Kim YK, Won JY, Chung JW. Chemoembolization with Vascular Disrupting Agent CKD-516 Dissolved in Ethiodized Oil in Combination with Doxorubicin: A VX2 Tumor Model Study. J Vasc Interv Radiol. 2018;29:1078-84
148. Dings RP, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH. et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13:3395-402
Author contact
![]() Corresponding author: (E-mail) jhryure.kr; ikwonre.kr
Corresponding author: (E-mail) jhryure.kr; ikwonre.kr
 Global reach, higher impact
Global reach, higher impact