13.3
Impact Factor
Theranostics 2020; 10(1):123-132. doi:10.7150/thno.37728 This issue Cite
Research Paper
A Microfluidic Platform for Single Cell Fluorometric Granzyme B Profiling
1. Graduate School of Engineering, Osaka University, Suita, Osaka, 565-0871, JAPAN.
2. Graduate School of Medicine, Osaka University, Suita, Osaka, 565-0871, JAPAN.
3. AIST PhotoBIO-OIL, Osaka University, Suita, Osaka, 565-0871, JAPAN.
* These authors contributed equally to this work.
Abstract
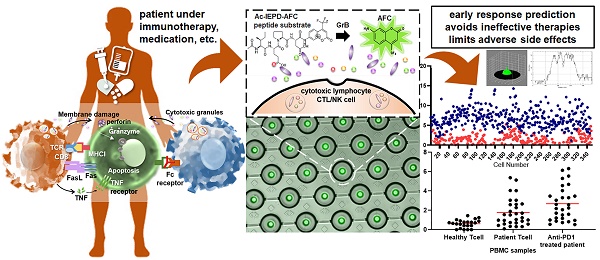
Granzyme B (GrB) is an essential cytotoxic effector in cancer immunotherapy as it can be a potential biomarker to predict the efficacy of immunotherapies including checkpoint inhibitors. Monitoring the Granzyme B activity in cells would help determine a patient's clinical response to treatment and lead to better treatment strategies by preventing administration of ineffective therapies and avoid adverse events resulting in a delay in subsequent treatment.
Methods: A microfluidic device with hydrodynamic traps and pneumatic valving system was fabricated using photo and soft lithography. Single cell Granzyme B (GrB) activity was detected and measured fluorometrically using a commercial assay kit with a peptide substrate containing GrB recognition sequence (Ac-IEPD-AFC) and AFC (7-Amino-4-trifluoromethylcoumarin) label. Fluorescence was observed and measured using a confocal microscope with CSU-W1 scanner unit and CCD camera as well as an inverted microscope with photodetector. Model cells (NK-92, GrB-transduced Jurkat, and THP1 cells) and human PBMCs from healthy donor and lung cancer patients including an anti-PD-1 antibody treated patient were profiled of its GrB activity as proof of concept.
Results: GrB expression from the model cells was found to be markedly different. NK-92 cells were found to have higher GrB activity than the GrB-transduced Jurkat cells. THP-1 was found to have relatively no significant activity. A marked increase in GrB expression was also observed in anti-PD-1 treated lung cancer patient sample in comparison to PBMC from a healthy donor. TCR+ Ig-G4+ PBMC cells were found to have high activity which signifies a clear response to PD-1 blockade.
Conclusion: As proof of concept, we have shown the capability of a microfluidic platform to measure GrB production through a single cell enzymatic activity assay. Our platform might be a promising tool for evaluating the sensitivity of immunotherapies and identifying specific T cell subset responsible for the anti-tumor response.
Keywords: single cell, granzyme b profiling, microfluidics, immunotherapy
 Global reach, higher impact
Global reach, higher impact