13.3
Impact Factor
Theranostics 2020; 10(22):10154-10170. doi:10.7150/thno.45861 This issue Cite
Research Paper
TRPM8-regulated calcium mobilization plays a critical role in synergistic chemosensitization of Borneol on Doxorubicin
1. Department of Cell Biology & Institute of Biomedicine, National Engineering Research Center of Genetic Medicine, Guangdong Provincial Key Laboratory of Bioengineering Medicine, College of Life Science and Technology, Jinan University, Guangzhou, 510632, China.
2. Department of Chemistry, Jinan University, Guangzhou, 510632, China.
Abstract
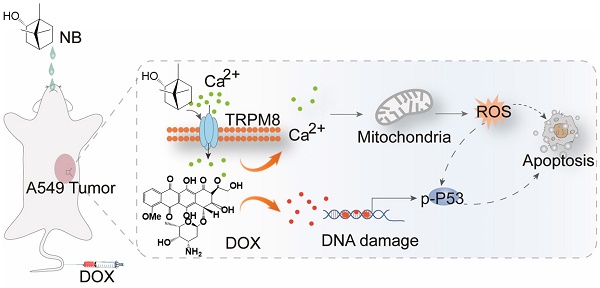
Background: Lung cancer has a high mortality rate and is resistant to multiple chemotherapeutics. Natural Borneol (NB) is a monoterpenoid compound that facilitates the bioavailability of drugs. In this study, we investigated the effects of NB on chemosensitivity in the A549 human lung adenocarcinoma cell line and to elucidate therapeutic molecular target of NB.
Methods: The chemosensitivity effects of NB in A549 cells were examined by MTT assay. The mechanism of NB action was evaluated using flow cytometry and Western blotting assays. Surface plasmon resonance (SPR) and LC-MS combined analysis (MS-SPRi) was performed to elucidate the candidate molecular target of NB. The chemosensitizing capacity of NB in vivo was assessed in nude mice bearing A549 tumors.
Results: NB pretreatment sensitized A549 cells to low doxorubicin (DOX) dosage, leading to a 15.7% to 41.5% increase in apoptosis. This increase was correlated with ERK and AKT inactivation and activation of phospho-p38 MAPK, phospho-JNK, and phosphor-p53. Furthermore, this synergism depends on reactive oxygen species (ROS) generation. MS-SPRi analysis revealed that transient receptor potential melastatin-8 (TRPM8) is the candidate target of NB in potentiating DOX killing potency. Genetically, TRPM8 knock-down significantly suppresses the chemosensitizing effects of NB and inhibits ROS generation through restraining calcium mobilization. Moreover, pretreatment with NB synergistically enhances the anticancer effects of DOX to delay tumor progression in vivo.
Conclusions: These results suggest that TRPM8 may be a valid therapeutic target in the potential application of NB, and show that NB is a chemosensitizer for lung cancer treatment.
Keywords: Natural Borneol, DOX, TRPM8, Synergism, Calcium mobilization, Non-small cell lung cancer
 Global reach, higher impact
Global reach, higher impact