13.3
Impact Factor
Theranostics 2020; 10(22):10245-10261. doi:10.7150/thno.47045 This issue Cite
Research Paper
Histone deacetylase inhibitors promote epithelial-mesenchymal transition in Hepatocellular Carcinoma via AMPK-FOXO1-ULK1 signaling axis-mediated autophagy
1. Department of Clinical Laboratory, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, China.
2. Cancer Hospital and Cancer Research Institute, Guangzhou Medical University, Guangzhou, Guangdong, China.
3. Department of Microbial and Biochemical Pharmacy, School of Pharmaceutical Sciences of Sun Yat-sen University, Guangzhou, Guangdong, China.
4. Department of Clinical Laboratory, The First People's Hospital of Changde City, Changde, Hunan, China.
5. Department of Clinical Laboratory, The First Affiliated Hospital of the University of South China, Hengyang, Hunan, China.
6. Department of Health Management, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, China.
7. Guangdong Provincial Key Laboratory of Biomedical Imaging, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, China.
8. Central Laboratory, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, China.
*These authors contributed equally to this work.
Abstract
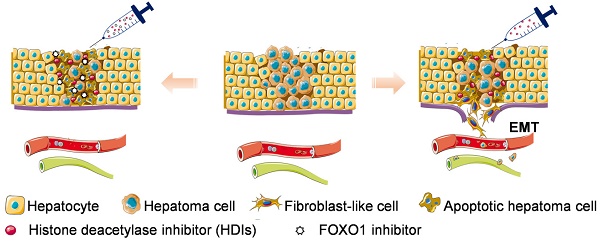
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer-related deaths globally because of high metastasis and recurrence rates. Elucidating the molecular mechanisms of HCC recurrence and metastasis and developing effective targeted therapies are expected to improve patient survival. The promising anti-cancer agents for the treatment of hematological malignancies, histone deacetylase inhibitors (HDIs), have limited effects against epithelial cell-derived cancers, including HCC, the mechanisms involved have not been elucidated. Herein, we studied the molecular mechanisms underlying HDI-induced epithelial-mesenchymal transition (EMT) involving FOXO1-mediated autophagy.
Methods: The biological functions of HDIs in combination with autophagy inhibitors were examined both in vitro and in vivo. Cell autophagy was assessed using the generation of mRFP-GFP-LC3-expressing cells and fluorescent LC3 puncta analysis, Western blotting, and electron microscopy. An orthotopic hepatoma model was established in mice for the in vivo experiments.
Results: Our study provided novel mechanistic insights into HDI-induced EMT mediated by the autophagy AMPK-FOXO1-ULK1-Snail signaling axis. We demonstrated that autophagy served as a pro-metastasis mechanism in HDI-treated hepatoma cells. HDIs induced autophagy via a FOXO1-dependent pathway, and FOXO1 inhibition promoted HDI-mediated apoptosis in hepatoma cells. Thus, our findings provided novel insights into the molecular mechanisms underlying HDI-induced EMT involving FOXO1-mediated autophagy and demonstrated that a FOXO1 inhibitor exerted a synergistic effect with an HDI to inhibit cell growth and metastasis in vitro and in vivo.
Conclusion: We demonstrated that HDIs triggers FOXO1-dependent autophagy, which ultimately promotes EMT, limiting the clinical outcome of HDI-based therapies. Our study suggests that the combination of an HDI and a FOXO1 inhibitor is an effective therapeutic strategy for the treatment of HCC.
Keywords: Histone deacetylase inhibitors, Autophagy, Epithelial-mesenchymal transition, FOXO1 inhibitor, Metastasis
 Global reach, higher impact
Global reach, higher impact