13.3
Impact Factor
Theranostics 2020; 10(23):10712-10728. doi:10.7150/thno.46143 This issue Cite
Research Paper
M2 macrophage-derived exosomes promote the c-KIT phenotype of vascular smooth muscle cells during vascular tissue repair after intravascular stent implantation
1. Key Laboratory of Bio-Rheological Science and Technology, State and Local Joint Engineering Laboratory for Vascular Implants, Bioengineering College of Chongqing University, Chongqing, China.
2. GIOME, Department of Surgery, the Chinese University of Hong Kong, Hong Kong, China.
3. The Nanoscience Centre, University of Cambridge, Cambridge, UK.
4. Cavendish Laboratory, University of Cambridge, Cambridge, UK.
Abstract
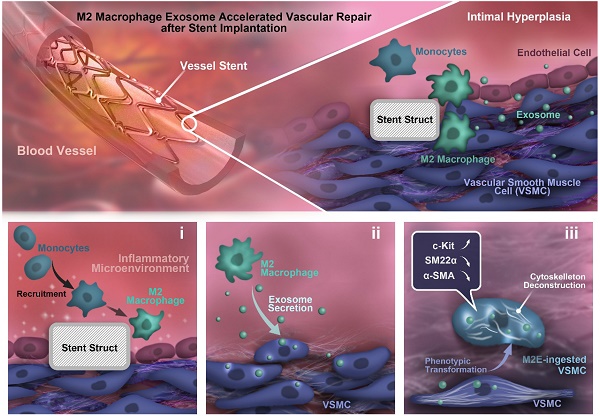
Rationale: For intravascular stent implantation to be successful, the processes of vascular tissue repair and therapy are considered to be critical. However, the mechanisms underlying the eventual fate of vascular smooth muscle cells (VSMCs) during vascular tissue repair remains elusive. In this study, we hypothesized that M2 macrophage-derived exosomes to mediate cell-to-cell crosstalk and induce dedifferentiation phenotypes in VSMCs.
Methods: In vivo, 316L bare metal stents (BMS) were implanted from the left iliac artery into the abdominal aorta of 12-week-old male Sprague-Dawley (SD) rats for 7 and 28 days. Hematoxylin and eosin (HE) were used to stain the neointimal lesions. En-face immunofluorescence staining of smooth muscle 22 alpha (SM22α) and CD68 showed the rat aorta smooth muscle cells (RASMCs) and macrophages. Immunohistochemical staining of total galactose-specific lectin 3 (MAC-2) and total chitinase 3-like 3 (YM-1) showed the total macrophages and M2 macrophages. In vitro, exosomes derived from IL-4+IL-13-treated macrophages (M2Es) were isolated by ultracentrifugation and characterized based on their specific morphology. Ki-67 staining was conducted to assess the effects of the M2Es on the proliferation of RASMCs. An atomic force microscope (AFM) was used to detect the stiffness of the VSMCs. GW4869 was used to inhibit exosome release. RNA-seq was performed to determine the mRNA profiles of the RASMCs and M2Es-treated RASMCs. Quantitative real-time PCR (qRT-PCR) analysis was conducted to detect the expression levels of the mRNAs. Western blotting was used to detect the candidate protein expression levels. T-5224 was used to inhibit the DNA binding activity of AP-1 in RASMCs.
Results: M2Es promote c-KIT expression and softening of nearby VSMCs, hence accelerating the vascular tissue repair process. VSMCs co-cultured in vitro with M2 macrophages presented an increased capacity for de-differentiation and softening, which was exosome dependent. In addition, the isolated M2Es helped to promote VSMC dedifferentiation and softening. Furthermore, the M2Es enhanced vascular tissue repair potency by upregulation of VSMCs c-KIT expression via activation of the c-Jun/activator protein 1 (AP-1) signaling pathway.
Conclusions: The findings of this study emphasize the prominent role of M2Es during VSMC dedifferentiation and vascular tissue repair via activation of the c-Jun/AP-1 signaling pathway, which has a profound impact on the therapeutic strategies of coronary stenting techniques.
Keywords: M2 macrophage, exosomes, stiffness, vascular smooth muscle cell, dedifferentiation, intravascular stent
 Global reach, higher impact
Global reach, higher impact