13.3
Impact Factor
Theranostics 2020; 10(23):10769-10790. doi:10.7150/thno.46878 This issue Cite
Research Paper
A feedforward circuit shaped by ECT2 and USP7 contributes to breast carcinogenesis
1. State Key Laboratory of Experimental Hematology, 2011 Collaborative Innovation Center of Tianjin for Medical Epigenetics, Key Laboratory of Immune Microenvironment and Disease (Ministry of Education), Key Laboratory of Breast Cancer Prevention and Therapy (Ministry of Education), Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University General Hospital, Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China.
2. Qingdao Haici Medical Treatment Group, Qingdao, 266000, China.
#These authors contributed equally to this work.
Abstract
Rationale: A number of guanine nucleotide exchange factors (GEFs) including epithelial cell transforming factor ECT2 are believed to drive carcinogenesis through activating distinct oncogenic GTPases. Yet, whether GEF-independent activity of ECT2 also plays a role in tumorigenesis remains unclear.
Methods: Immunohistochemical (IHC) staining, colony formation and xenograft assays were used to examine the role of ECT2 in breast carcinogenesis. Co-immunoprecipitation, immunofluorescent stainings, in vivo deubiquitination and in vitro deubiquitination experiments were performed to examine the physical and functional interaction between ECT2 and ubiquitin-specific protease USP7. High-throughput RNA sequencing, quantitative reverse transcription-PCR and Western blotting were employed to investigate the biological significance of the interplay between ECT2 and USP7.
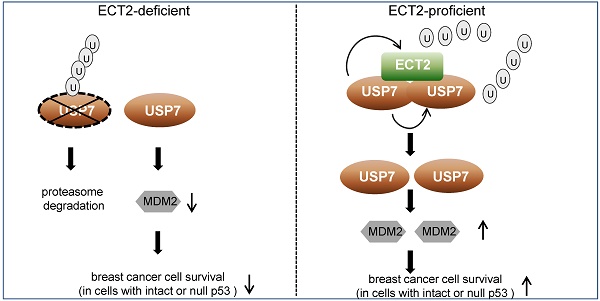
Results: We report that ECT2 plays a tumor-promoting role in breast cancer, and GEF activity-deficient ECT2 is able to alleviate ECT2 depletion associated growth defects in breast cancer cells. Mechanistically, we demonstrated that ECT2 physically interacts with ubiquitin-specific protease USP7 and functionally facilitates USP7 intermolecular self-association, -deubiquitination and -stabilization in a GEF activity-independent manner. USP7 in turn, deubiquitinates and stabilizes ECT2, resulting in a feedforward regulatory circuit that ultimately sustains the expression of oncogenic protein MDM2.
Conclusion: Our study uncovers a GEF-independent role of ECT2 in promoting survival of breast cancer cells, provides a molecular insight for the reciprocal regulation of ECT2 and USP7, and supports the pursuit of ECT2/USP7 as potential targets for breast cancer intervention.
Keywords: Guanine nucleotide exchange factors, Deubiquitination, Deubiquitinase, Protein stability, Breast cancer
 Global reach, higher impact
Global reach, higher impact