13.3
Impact Factor
Theranostics 2020; 10(24):10861-10873. doi:10.7150/thno.45440 This issue Cite
Research Paper
Pharmacological inhibition of mTORC1 increases CCKBR-specific tumor uptake of radiolabeled minigastrin analogue [177Lu]Lu-PP-F11N
1. Center for Radiopharmaceutical Sciences, Paul Scherrer Institute, Villigen, Switzerland.
2. Division of Neuropathology, Institute of Pathology, University of Basel, Switzerland.
3. Laboratory of Nanoscale Biology, Paul Scherrer Institute, Villigen, Switzerland.
4. Department of Chemistry and Applied Biosciences, ETH Zurich, Switzerland.
Abstract
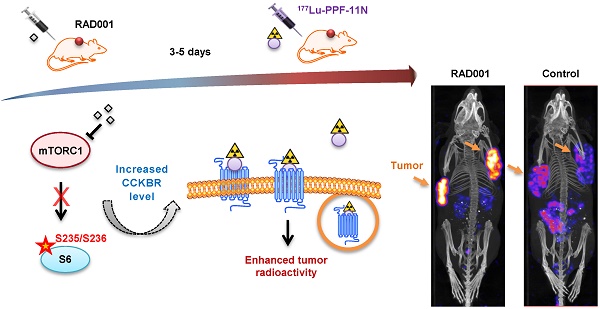
Rationale: A high tumor-to-healthy-tissue uptake ratio of radiolabeled ligands is an essential prerequisite for safe and effective peptide receptor radionuclide therapy (PRRT). In the present study, we searched for novel opportunities to increase tumor-specific uptake of the radiolabeled minigastrin analogue [177Lu]Lu-DOTA-(DGlu)6-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 ([177Lu]Lu-PP-F11N), that targets the cholecystokinin B receptor (CCKBR) in human cancers.
Methods: A kinase inhibitor library screen followed by proliferation and internalization assays were employed to identify compounds which can increase uptake of [177Lu]Lu-PP-F11N in CCKBR-transfected human epidermoid carcinoma A431 cells and natural CCKBR-expressing rat pancreatic acinar AR42J cells. Western blot (WB) analysis verified the inhibition of the signaling pathways and the CCKBR level, whereas the cell-based assay analyzed arrestin recruitment. Biodistribution and SPECT imaging of the A431/CCKBR xenograft mouse model as well as histological analysis of the dissected tumors were used for in vivo validation.
Results: Our screen identified the inhibitors of mammalian target of rapamycin complex 1 (mTORC1), which increased cell uptake of [177Lu]Lu-PP-F11N. Pharmacological mTORC1 inhibition by RAD001 and metformin increased internalization of [177Lu]Lu-PP-F11N in A431/CCKBR and in AR42J cells. Analysis of protein lysates from RAD001-treated cells revealed increased levels of CCKBR (2.2-fold) and inhibition of S6 phosphorylation. PP-F11N induced recruitment of β-arrestin1/2 and ERK1/2 phosphorylation. In A431/CCKBR-tumor bearing nude mice, 3 or 5 days of RAD001 pretreatment significantly enhanced tumor-specific uptake of [177Lu]Lu-PP-F11N (ratio [RAD001/Control] of 1.56 or 1.79, respectively), whereas metformin treatment did not show a significant difference. Quantification of SPECT/CT images confirmed higher uptake of [177Lu]Lu-PP-F11N in RAD001-treated tumors with ratios [RAD001/Control] of average and maximum concentration reaching 3.11 and 3.17, respectively. HE staining and IHC of RAD001-treated tumors showed a significant increase in necrosis (1.4% control vs.10.6% of necrotic area) and the reduction of proliferative (80% control vs. 61% of Ki67 positive cells) and mitotically active cells (1.08% control vs. 0.75% of mitotic figures). No significant difference in the tumor vascularization was observed after five-day RAD001 or metformin treatment.
Conclusions: Our data demonstrates, that increased CCKBR protein level by RAD001 pretreatment has the potential to improve tumor uptake of [177Lu]Lu-PP-F11N and provides proof-of-concept for the development of molecular strategies aimed at enhancing the level of the targeted receptor, to increase the efficacy of PRRT and nuclear imaging.
Keywords: Cholecystokinin B receptor, minigastrin, PPF-11N, RAD001, peptide receptor radionuclide therapy
 Global reach, higher impact
Global reach, higher impact