13.3
Impact Factor
Theranostics 2020; 10(3):1245-1261. doi:10.7150/thno.40060 This issue Cite
Research Paper
Promotion of mitochondrial fusion protects against developmental PBDE-47 neurotoxicity by restoring mitochondrial homeostasis and suppressing excessive apoptosis
1. Department of Occupational and Environmental Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People's Republic of China
2. Key Laboratory of Environment and Health, Ministry of Education, State Key Laboratory of Environmental health (incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People's Republic of China
3. Department of Clinical Nutrition, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People's Republic of China
* These authors contributed equally to this work.
Abstract
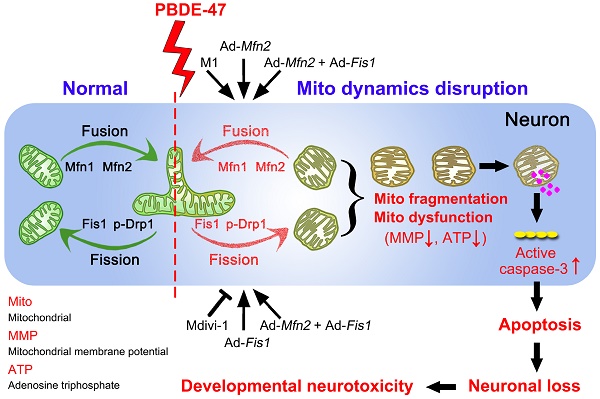
Polybrominated diphenyl ethers (PBDEs)-induced neurotoxicity is closely associated with mitochondrial abnormalities. Mitochondrial fusion and fission dynamics are required for the maintenance of mitochondrial homeostasis. However, little is known about how PBDEs disrupt this dynamics and whether such disruption contributes to impaired neurodevelopment.
Methods: We investigated the effects of 2, 2', 4, 4'-tetrabromodiphenyl ether (PBDE-47), the dominant congener in human samples, on mitochondrial fusion and fission dynamics using PC12 cells, a well-defined in vitro neurodevelopmental model. We also evaluated the effects of perinatal low-dose PBDE-47 exposure on hippocampal mitochondrial dynamics and its association with neurobehavioral changes in adult Sprague-Dawley rats.
Results: In vitro, PBDE-47 disrupted mitochondrial dynamics by inhibiting mitochondrial fusion and fission simultaneously, accompanied by mitochondrial fragmentation, membrane potential dissipation, ATP loss, and apoptosis activation. Specifically, enhancing mitochondrial fusion by the chemical promoter M1 or adenovirus-mediated mitofusin 2 (Mfn2) overexpression rescued PBDE-47-caused mitochondrial dynamic, morphological and functional impairments, prevented the resultant apoptosis and promoted neuronal survival. Unexpectedly, either stimulating mitochondrial fission by adenovirus-mediated fission protein 1 (Fis1) overexpression or suppressing mitochondrial fission by the mitochondrial division inhibitor-1 (Mdivi-1) failed to reverse whereas aggravated PBDE-47-induced mitochondrial damage and neuronal death. Importantly, promoting mitochondrial fusion by Mfn2 overexpression neutralized the detrimental effects elicited by Fis1 overexpression after PBDE-47 treatment. Finally, perinatal oral administration of PBDE-47 elicited neurobehavioral deficits and hippocampal neuronal loss via apoptosis in adult rats, which were associated with mitochondrial dynamics alterations manifested as a fragmented phenotype.
Conclusion: Our results suggest that PBDE-47 disrupts mitochondrial dynamics to induce mitochondrial abnormalities, triggering apoptosis and thus contributing to neuronal loss and subsequent neurobehavioral deficits. Targeting mitochondrial fusion may be a promising therapeutic intervention against PBDE-47 neurotoxicity.
Keywords: PBDE-47, developmental neurotoxicity, mitochondrial fusion and fission, mitochondrial dysfunction, apoptosis.
 Global reach, higher impact
Global reach, higher impact