13.3
Impact Factor
Theranostics 2020; 10(3):1319-1331. doi:10.7150/thno.37543 This issue Cite
Review
EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles
1. Univ Lyon Université Claude Bernard Lyon 1, CNRS, Institut Lumière Matière, Lyon, France.
2. NH TherAguix SA, Meylan, France.
3. Institut Universitaire de France.
4. Institut des Sciences Moléculaires, CNRS UMR 5255, Université de Bordeaux, Bordeaux, France.
5. Radiotherapy department, CHU de Grenoble, Grenoble cedex 9, France.
6. Synchrotron Radiation for Biomedical Research Inserm UA7, University of Grenoble Alps, France.
Abstract
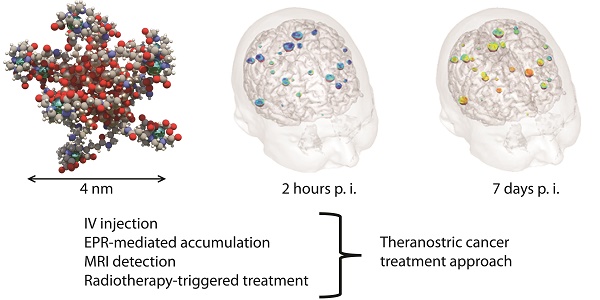
Interest of tumor targeting through EPR effect is still controversial due to intrinsic low targeting efficacy and rare translation to human cancers. Moreover, due to different reasons, it has generally been described for relatively large nanoparticles (NPs) (hydrodynamic diameter > 10 nm). In this review EPR effect will be discussed for ultrasmall NPs using the example of the AGuIX® NP (Activation and Guiding of Irradiation by X-ray) recently translated in clinic. AGuIX® NP is a 4 ± 2 nm hydrodynamic diameter polysiloxane based NP. Since AGuIX® NP biodistribution is monitored by magnetic resonance imaging (MRI) and its activation is triggered by irradiation upon X-rays, this NP is well adapted for a theranostic approach of radiotherapy cancer treatment. Here we show that AGuIX® NP is particularly well suited to benefit from EPR-mediated tumor targeting thanks to an ultrasmall size and efficacy under irradiation at small dose. Indeed, intravenously-injected AGuIX® NP into rodent cancer models passively reached the tumor and revealed no toxicity, favoured by renal clearance. Moreover, translation of AGuIX® NP accumulation and retention into humans carrying brain metastases was validated during a first-in-man phase Ib trial taking advantage of easy biodistribution monitoring by MRI.
Keywords: theranostic, ultrasmall nanoparticle, EPR effect, clinical translation, AGuIX
 Global reach, higher impact
Global reach, higher impact