13.3
Impact Factor
Theranostics 2020; 10(3):1373-1390. doi:10.7150/thno.39157 This issue Cite
Research Paper
Potent antibacterial activity of MSI-1 derived from the magainin 2 peptide against drug-resistant bacteria
School of Life Science and Technology, China Pharmaceutical University, Nanjing, Jiangsu 211198, PR China
*These authors contributed equally to this work.
Abstract
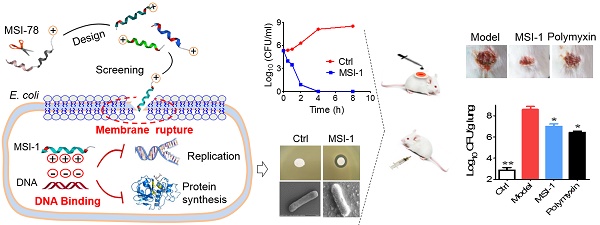
The structural modification of existing AMPs is an effective strategy to develop antimicrobial agents with high-efficiency, low-cost and low-toxicity antimicrobial agents.
Methods: Here, we truncated 14-amino-acids at the N-terminus of MSI-78 to obtain MSI and further modified MSI to obtain four peptide analogs: MSI-1, MSI-2, MSI-3 and MSI-4. These peptide mutants were evaluated regarding their antibacterial activity against various sensitive or resistant bacteria; toxicity against mammalian cells or mice; and stability against violent pH, temperature variations and high NaCl concentrations. Finally, we also elucidated the possible mechanisms underlying its mode of action.
Results: The results showed that MSI-1 and MSI-3 displayed activity that was superior to that of MSI-78 with MICs of 4-16 μg/ml and MBCs of 8-64 μg/ml, respectively, especially against drug-resistant bacteria, due to the increase in percent helicity and amphiphilicity. However, MSI-3, with higher hydrophobicity and antibacterial activity, had a relatively higher hemolysis rate and toxicity than MSI-1. MSI-1 exerted rapid bactericidal activity and effectively improved the survival rate and wound closure in penicillin-resistant E. coli-infected mice by eliminating bacterial counts in mouse organs or subeschar, further inhibiting the systemic dissemination of bacteria. Additionally, MSI-1 displayed perfect stability against violent pH, temperature variations and high NaCl concentrations and has the ability to circumvent the development of drug resistance. In terms of the mode of action, we found that at the super-MIC level, MSI-1 exhibited direct antimicrobial activity by disrupting the integrity of the bacterial cell membrane, while at the sub-MIC level, it bound to bacterial DNA to inhibit DNA replication and protein expression and ultimately disrupted bacterial biological function.
Conclusions: This novel peptide MSI-1 could be a potential candidate for drug development against infection induced by drug-resistant bacteria.
Keywords: AMPs structural modification, MSI-1, antibacterial activity, drug-resistant bacteria, membrane rupture, DNA binding
 Global reach, higher impact
Global reach, higher impact