13.3
Impact Factor
Theranostics 2020; 10(4):1746-1757. doi:10.7150/thno.39089 This issue Cite
Research Paper
The Influence of Glycans-Specific Bioconjugation on the FcγRI Binding and In vivo Performance of 89Zr-DFO-Pertuzumab
1. Department of Chemistry, Hunter College, City University of New York, New York, NY, USA
2. Ph.D. Program in Chemistry, The Graduate Center of the City University of New York, New York, NY, USA
3. Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
4. Department of Radiology, Weill Cornell Medical College, Center, New York, NY, USA
5. Program in Molecular Pharmacology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
*These two authors contributed equally to this work.
#Current address: Institut de Chimie Moléculaire de l'Université de Bourgogne, Dijon, France
Abstract
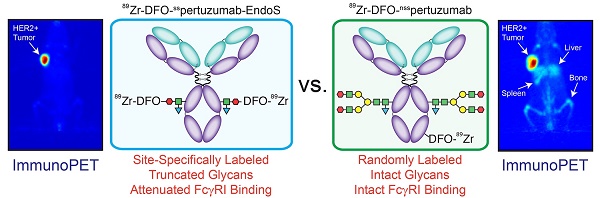
Rationale: The overwhelming majority of radioimmunoconjugates are produced via random conjugation methods predicated on attaching bifunctional chelators to the lysines of antibodies. However, this approach inevitably produces poorly defined and heterogeneous immunoconjugates because antibodies have several lysines distributed throughout their structure. To circumvent this issue, we have previously developed a chemoenzymatic bioconjugation strategy that site-specifically appends cargoes to the biantennary heavy chain glycans attached to CH2 domains of the immunoglobulin's Fc region. In the study at hand, we explore the effects of this approach to site-specific bioconjugation on the Fc receptor binding and in vivo behavior of radioimmunoconjugates.
Methods: We synthesized three desferrioxamine (DFO)-labeled immunoconjugates based on the HER2-targeting antibody pertuzumab: one using random bioconjugation methods (DFO-nsspertuzumab) and two using variants of our chemoenzymatic protocol (DFO-sspertuzumab-EndoS and DFO-sspertuzumab-βGal). Subsequently, we characterized these constructs and evaluated their ability to bind HER2, human FcγRI (huFcγRI), and mouse FcγRI (muFcγRI). After radiolabeling the immunoconjugates with zirconium-89, we conducted PET imaging and biodistribution studies in two different mouse models of HER2-expressing breast cancer.
Results: MALDI-ToF and SDS-PAGE analysis confirmed the site-specific nature of the bioconjugation, and flow cytometry and surface plasmon resonance (SPR) revealed that all three immunoconjugates bind HER2 as effectively as native pertuzumab. Critically, however, SPR experiments also illuminated that DFO-sspertuzumab-EndoS possesses an attenuated binding affinity for huFcγRI (17.4 ± 0.3 nM) compared to native pertuzumab (4.7 ± 0.2 nM), DFO-nsspertuzumab (4.1 ± 0.1 nM), and DFO-sspertuzumab-βGal (4.7 ± 0.2 nM). ImmunoPET and biodistribution experiments in athymic nude mice bearing HER2-expressing BT474 human breast cancer xenografts yielded no significant differences in the in vivo behavior of the radioimmunoconjugates. Yet experiments in tumor-bearing humanized NSG mice revealed that 89Zr-DFO-sspertuzumab-EndoS produces higher activity concentrations in the tumor (111.8 ± 39.9 %ID/g) and lower activity concentrations in the liver and spleen (4.7 ± 0.8 %ID/g and 13.1 ± 4.0 %ID/g, respectively) than its non-site-specifically labeled cousin, a phenomenon we believe stems from the altered binding of the former to huFcγRI.
Conclusion: These data underscore that this approach to site-specific bioconjugation not only produces more homogeneous and well-defined radioimmunoconjugates than traditional methods but may also improve their in vivo performance in mouse models by reducing binding to FcγRI.
Keywords: 89Zr, pertuzumab, HER2, site-specific, glycans, immunoPET, radioimmunoconjugate
 Global reach, higher impact
Global reach, higher impact