13.3
Impact Factor
Theranostics 2020; 10(5):2293-2308. doi:10.7150/thno.39238 This issue Cite
Research Paper
Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism
1. Department of Orthopedics, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China.
2. Movement System Injury and Repair Research Center, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China.
3. Department of Metabolism and Endocrinology, The First Affiliated Hospital, University of South China, Hengyang, Hunan 421001, China.
4. Department of Sports Medicine, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China.
5. Xiangya Nursing School, Central South University, Changsha, Hunan 410013, China.
6. Department of Forensic Science, School of Basic Medical Science, Central South University, Changsha, Hunan 410013, China.
7. Hunan Key Laboratory of Organ Injury, Aging and Regenerative Medicine, Changsha, Hunan 410008, China.
8. Hunan Key Laboratory of Bone Joint Degeneration and Injury, Changsha, Hunan 410008, China.
9. National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China.
*Yin Hu and Yan Zhang contributed equally to this work.
Abstract
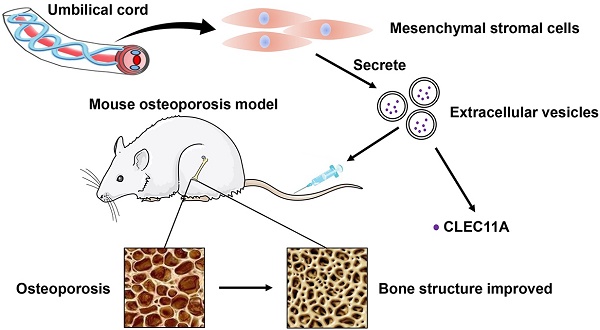
Osteoporosis and osteoporotic fractures severely compromise quality of life in elderly people and lead to early death. Human umbilical cord mesenchymal stromal cell (MSC)-derived extracellular vesicles (hucMSC-EVs) possess considerable therapeutic effects in tissue repair and regeneration. Thus, in the present study, we investigated the effects of hucMSC-EVs on primary and secondary osteoporosis and explored the underlying mechanisms.
Methods: hucMSCs were isolated and cultured. EVs were obtained from the conditioned medium of hucMSCs and determined by using transmission electron microscopy, dynamic light scattering and Western Blot analyses. The effects of hucMSC-EVs on ovariectomy-induced postmenopausal osteoporosis and tail suspension-induced hindlimb disuse osteoporosis in mouse models were assessed by using microcomputed tomography, biomechanical, histochemical and immunohistochemical, as well as histomorphometric analyses. Proteomic analysis was applied between hucMSC-EVs and hucMSCs to screen the candidate proteins that mediate hucMSC-EVs function. The effects of hucMSC-EVs on osteogenic and adipogenic differentiation of bone marrow mesenchymal stromal cells (BMSCs), and osteoclastogenesis of the macrophage cell line RAW264.7 in vitro were determined by using cytochemical staining and quantitative real-time PCR analysis. Subsequently, the roles of the key protein in hucMSC-EVs-induced regulation on BMSCs and RAW264.7 cells were evaluated.
Results: hucMSCs were able to differentiate into osteoblasts, adipocytes or chondrocytes and positively expressed CD29, CD44, CD73 and CD90, but negatively expressed CD34 and CD45. The morphological assessment revealed the typical cup- or sphere-shaped morphology of hucMSC-EVs with diameters predominantly ranging from 60 nm to 150 nm and expressed CD9, CD63, CD81 and TSG101. The systemic administration of hucMSC-EVs prevented bone loss and maintained bone strength in osteoporotic mice by enhancing bone formation, reducing marrow fat accumulation and decreasing bone resorption. Proteomic analysis showed that the potently pro-osteogenic protein, CLEC11A (C-type lectin domain family 11, member A) was very highly enriched in hucMSC-EVs. In addition, hucMSC-EVs enhanced the shift from adipogenic to osteogenic differentiation of BMSCs via delivering CLEC11A in vitro. Moreover, CLEC11A was required for the inhibitory effects of hucMSC-EVs on osteoclast formation.
Conclusion: Our results suggest that hucMSC-EVs serve as a critical regulator of bone metabolism by transferring CLEC11A and may represent a potential agent for prevention and treatment of osteoporosis.
Keywords: extracellular vesicles, umbilical cord-derived mesenchymal stromal cells, bone homeostasis, osteoporosis, CLEC11A
 Global reach, higher impact
Global reach, higher impact