13.3
Impact Factor
Theranostics 2020; 10(6):2597-2611. doi:10.7150/thno.40595 This issue Cite
Research Paper
Coronary artery mechanics induces human saphenous vein remodelling via recruitment of adventitial myofibroblast-like cells mediated by Thrombospondin-1
1. Unità di Ingegneria Tissutale Cardiovascolare; Centro Cardiologico Monzino, IRCCS; Milan, Italy
2. PhD Program in Translational and Molecular Medicine - DIMET; Università di Milano - Bicocca, Milan, Italy
3. Dipartimento di Elettronica, Informazione e Bioingegneria; Politecnico di Milano; Milan, Italy
4. Unità di Proteomica; Centro Cardiologico Monzino, IRCCS, Milan, Italy
5. Bristol Heart Institute, University of Bristol; Bristol, UK
6. Dipartimento di Scienze Cliniche e di Comunità; Università di Milano, Milan, Italy; Centro Cardiologico Monzino, IRCCS, Milan, Italy
7. Unità di Chirurgia Vascolare, Centro Cardiologico Monzino, IRCCS; Milan, Italy
8. IRCCS Multimedica, Milan, Italy
*Equal contribution
Abstract
Rationale: Despite the preferred application of arterial conduits, the greater saphenous vein (SV) remains indispensable for coronary bypass grafting (CABG), especially in multi-vessel coronary artery disease (CAD). The objective of the present work was to address the role of mechanical forces in the activation of maladaptive vein bypass remodeling, a process determining progressive occlusion and recurrence of ischemic heart disease.
Methods: We employed a custom bioreactor to mimic the coronary shear and wall mechanics in human SV vascular conduits and reproduce experimentally the biomechanical conditions of coronary grafting and analyzed vein remodeling process by histology, histochemistry and immunofluorescence. We also subjected vein-derived cells to cyclic uniaxial mechanical stimulation in culture, followed by phenotypic and molecular characterization using RNA and proteomic methods. We finally validated our results in vitro and using a model of SV carotid interposition in pigs.
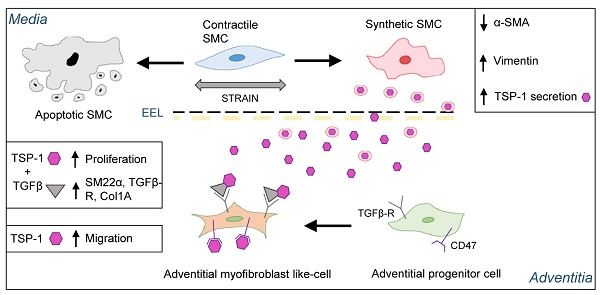
Results: Exposure to pulsatile flow determined a remodeling process of the vascular wall involving reduction in media thickness. Smooth muscle cells (SMCs) underwent conversion from contractile to synthetic phenotype. A time-dependent increase in proliferating cells expressing mesenchymal (CD44) and early SMC (SM22α) markers, apparently recruited from the SV adventitia, was observed especially in CABG-stimulated vessels. Mechanically stimulated SMCs underwent transition from contractile to synthetic phenotype. MALDI-TOF-based secretome analysis revealed a consistent release of Thrombospondin-1 (TSP-1), a matricellular protein involved in TGF-β-dependent signaling. TSP-1 had a direct chemotactic effect on SV adventitia resident progenitors (SVPs); this effects was inhibited by blocking TSP-1 receptor CD47. The involvement of TSP-1 in adventitial progenitor cells differentiation and graft intima hyperplasia was finally contextualized in the TGF-β-dependent pathway, and validated in a saphenous vein into carotid interposition pig model.
Conclusions: Our results provide the evidence of a matricellular mechanism involved in the human vein arterialization process controlled by alterations in tissue mechanics, and open the way to novel potential strategies to block VGD progression based on targeting cell mechanosensing-related effectors.
Keywords: coronary artery bypass grafting, vein graft disease, mechanotransduction, Thrombospondin-1, arterialization
 Global reach, higher impact
Global reach, higher impact