13.3
Impact Factor
Theranostics 2020; 10(6):2645-2658. doi:10.7150/thno.38533 This issue Cite
Research Paper
Nanobody-based CD38-specific heavy chain antibodies induce killing of multiple myeloma and other hematological malignancies
1. Department of Diagnostic and Interventional Radiology and Nuclear medicine, University Medical Center, Hamburg-Eppendorf, Germany.
2. Institute of Immunology, University Medical Center, Hamburg-Eppendorf, Germany.
3. Department of Neurology, University Medical Center, Hamburg-Eppendorf, Germany.
4. Research Department Cell and Gene Therapy, University Medical Center, Hamburg-Eppendorf, Germany.
5. Institute of Medical Biometry and Epidemiology, University Medical Center, Hamburg-Eppendorf, Germany.
6. Department of Stem Cell Transplantation, University Medical Center, Hamburg-Eppendorf, Germany.
7. Department of Oncology and Hematology, University Medical Center, Hamburg-Eppendorf, Germany.
8. Department of Haematology and Oncology, University Hospital Halle, Halle, Germany.
9. Hematology and Oncology Center Altona (HOPA), Hamburg, Germany.
Nanobody® is a trademark of Ablynx. In this paper we use nanobody as the generic term for the recombinant VHH domain of a llama heavy chain antibody.
* LS, KS, and KP contributed equally
# FK-N and PB share senior authorship
Abstract
Rationale: CD38 is a target for the therapy of multiple myeloma (MM) with monoclonal antibodies such as daratumumab and isatuximab. Since MM patients exhibit a high rate of relapse, the development of new biologics targeting alternative CD38 epitopes is desirable. The discovery of single-domain antibodies (nanobodies) has opened the way for a new generation of antitumor therapeutics. We report the generation of nanobody-based humanized IgG1 heavy chain antibodies (hcAbs) with a high specificity and affinity that recognize three different and non-overlapping epitopes of CD38 and compare their cytotoxicity against CD38-expressing hematological cancer cells in vitro, ex vivo and in vivo.
Methods: We generated three humanized hcAbs (WF211-hcAb, MU1067-hcAb, JK36-hcAb) that recognize three different non-overlapping epitopes (E1, E2, E3) of CD38 by fusion of llama-derived nanobodies to the hinge- and Fc-domains of human IgG1. WF211-hcAb shares the binding epitope E1 with daratumumab. We compared the capacity of these CD38-specific hcAbs and daratumumab to induce CDC and ADCC in CD38-expressing tumor cell lines in vitro and in patient MM cells ex vivo as well as effects on xenograft tumor growth and survival in vivo.
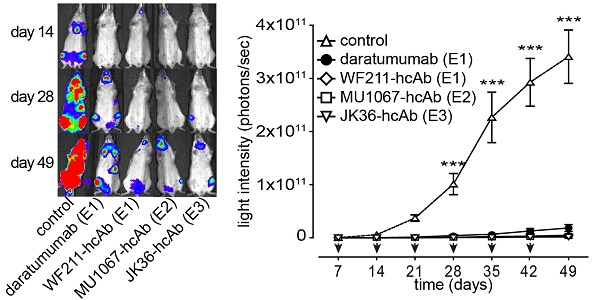
Results: CD38-specific heavy chain antibodies (WF211-hcAb, MU1067-hcAb, JK36-hcAb) potently induced antibody-dependent cellular cytotoxicity (ADCC) in CD38-expressing tumor cell lines and in primary patient MM cells, but only little if any complement-dependent cytotoxicity (CDC). In vivo, CD38-specific heavy chain antibodies significantly reduced the growth of systemic lymphomas and prolonged survival of tumor bearing SCID mice.
Conclusions: CD38-specific nanobody-based humanized IgG1 heavy chain antibodies mediate cytotoxicity against CD38-expressing hematological cancer cells in vitro, ex vivo and in vivo. These promising results of our study indicate that CD38-specific hcAbs warrant further clinical development as therapeutics for multiple myeloma and other hematological malignancies.
 Global reach, higher impact
Global reach, higher impact