13.3
Impact Factor
Theranostics 2020; 10(7):3138-3150. doi:10.7150/thno.43441 This issue Cite
Research Paper
LRR domain of NLRX1 protein delivery by dNP2 inhibits T cell functions and alleviates autoimmune encephalomyelitis
1. Department of Life Science, College of Natural Sciences, Hanyang University, Seoul 04763, Republic of Korea
2. Research Institute for Natural Sciences, Hanyang University, Seoul 04763, Republic of Korea
3. Section of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT 06520, USA
4. Research Institute for Convergence of Basic Sciences, Hanyang University, Seoul 04763, Republic of Korea
Abstract
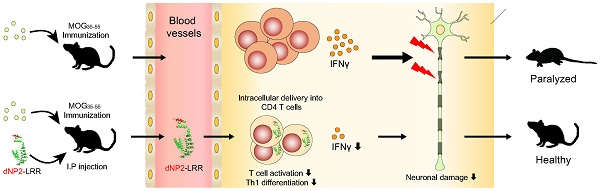
Multiple sclerosis (MS) is a demyelinating inflammatory disease of the central nervous system (CNS), which is a chronic progressive disease and is caused by uncontrolled activation of myelin antigen specific T cells. It has high unmet medical needs due to the difficulty of efficient drug delivery into the CNS to control tissue inflammation. In this study, we demonstrate that a fusion protein of NOD-like receptor family member X1 (NLRX1) and blood brain barrier (BBB)-permeable peptide, dNP2 ameliorates experimental autoimmune encephalomyelitis (EAE).
Methods: We purified recombinant LRR or NBD regions of NLRX1 protein conjugated with dNP2. To examine intracellular delivery efficiency of the recombinant protein, we incubated the proteins with Jurkat T cells or murine splenic T cells and their delivery efficiency was analyzed by flow cytometry. To investigate the therapeutic efficacy in an EAE model, we injected the recombinant protein into mice with 3 different treatment schemes e.g., prevention, semi-therapeutic, and therapeutic. To analyze their functional roles in T cells, we treated MACS-sorted naïve CD4 T cells with the proteins during their activation and differentiation into Th1, Th17, and Treg cells.
Results: dNP2-LRR protein treatment showed significantly higher delivery efficiency than TAT-LRR or LRR alone in Jurkat T cells and mouse splenic T cells. In all three treatment schemes of EAE experiments, dNP2-LRR administration showed ameliorated tissue inflammation and disease severity with reduced number of infiltrating T cells producing inflammatory cytokines such as IFNγ. In addition, dNP2-LRR inhibited T cell activation, cytokine production, and Th1 differentiation.
Conclusion: These results suggest that dNP2-LRR is a novel agent, which regulates effector T cell functions and could be a promising molecule for the treatment of CNS autoimmune diseases such as multiple sclerosis.
Keywords: NOD-like receptor family member X1, T cell, BBB-penetrating peptide, dNP2, experimental autoimmune encephalomyelitis
 Global reach, higher impact
Global reach, higher impact