13.3
Impact Factor
Theranostics 2020; 10(8):3397-3412. doi:10.7150/thno.42243 This issue Cite
Research Paper
Novel calreticulin-nanoparticle in combination with focused ultrasound induces immunogenic cell death in melanoma to enhance antitumor immunity
1. Center for Veterinary Health Sciences, Oklahoma State University, Stillwater, Oklahoma 74074
2. Geisel School of Medicine, Dartmouth, Hanover, NH03755
3. Albert Einstein College of Medicine, Bronx, New York 10461
# Both authors contributed equally to the experimental study and drafting of the manuscript.
Abstract
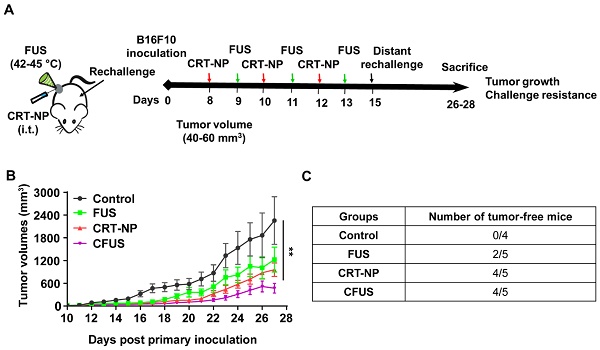
Rationale: Some studies have shown that the local activation of immunogenic cell death (ICD) by upregulating calreticulin (CRT) expression in solid tumors can improve antitumor effects. Although a promising approach, a key current challenge in ICD tumor therapy is the absence of a clinically translatable method for reproducibly inducing the CRT expression. Herein, we report a novel calreticulin-nanoparticle (CRT-NP) that enhances ICD and synergizes with focused ultrasound (FUS) to achieve local and systemic antitumor effects.
Methods: Full-length clone DNA of calreticulin was encapsulated in NPs made from DOTAP and cholesterol. Three CRT-NP intratumoral injections of 20 µg each were given 2 days apart, and FUS heating (42-45°C, ~15min) was applied sequentially 24h after each injection to induce ICD. To investigate ICD specific immune effect, the splenocytes of mice vaccinated with CRT-NP (± FUS) treated B16F10 cells were evaluated ex-vivo for TRP-2 antigen specific immunity. Additionally, the long-term protection was evaluated by re-challenging with the melanoma cells in the flank regions of tumor bearing mice.
Results: CRT-NP plus FUS (CFUS) upregulated CRT expression, expanded the population of melanoma TRP-2 specific functional CD4+ and CD8+ T cells and tumor-suppressing M1 phenotype, and increased PD-1 and PD-L1 marker expression in the T cells. Therapeutically, CFUS suppressed B16 melanoma growth by >85% vs. that seen in untreated controls, and >~50% vs. CRT-NP or FUS alone, and prevented tumor growth in distal untreated sites.
Conclusions: CRT-NP amplifies the FUS and ICD therapeutic outcomes against melanoma, suggesting that the proposed combinatorial methodology may be clinically translatable.
Keywords: Immunogenic cell death, calreticulin, nanoparticle, melanoma, focused ultrasound
 Global reach, higher impact
Global reach, higher impact