13.3
Impact Factor
Theranostics 2020; 10(9):4233-4249. doi:10.7150/thno.40664 This issue Cite
Research Paper
Loss of EGR-1 uncouples compensatory responses of pancreatic β cells
1. Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Taiwan, ROC;
2. Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, Taiwan, ROC;
3. Department of Physiology, College of Medicine, National Cheng Kung University, Taiwan, ROC;
4. Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Taiwan, ROC;
5. Department of Pharmacology, College of Medicine, National Cheng Kung University, Taiwan, ROC;
6. Department of Medical Laboratory Science and Biotechnology, College of Medicine, National Cheng Kung University, Taiwan, ROC;
7. School of Medicine, College of Medicine, I-Shou University, Kaohsiung, Taiwan, ROC;
8. Center for Clinical Medicine Research, National Cheng Kung University Hospital, Tainan, Taiwan, ROC
9. Department of Surgery, National Cheng Kung University Hospital, Tainan, Taiwan, ROC
10. Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan, Taiwan, ROC
*Contributed equally to this work
Abstract
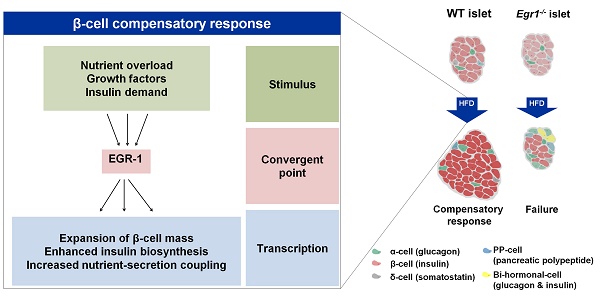
Rationale: Subjects unable to sustain β-cell compensation develop type 2 diabetes. Early growth response-1 protein (EGR-1), implicated in the regulation of cell differentiation, proliferation, and apoptosis, is induced by diverse metabolic challenges, such as glucose or other nutrients. Therefore, we hypothesized that deficiency of EGR-1 might influence β-cell compensation in response to metabolic overload.
Methods: Mice deficient in EGR-1 (Egr1-/-) were used to investigate the in vivo roles of EGR-1 in regulation of glucose homeostasis and beta-cell compensatory responses.
Results: In response to a high-fat diet, Egr1-/- mice failed to secrete sufficient insulin to clear glucose, which was associated with lower insulin content and attenuated hypertrophic response of islets. High-fat feeding caused a dramatic impairment in glucose-stimulated insulin secretion and downregulated the expression of genes encoding glucose sensing proteins. The cells co-expressing both insulin and glucagon were dramatically upregulated in islets of high-fat-fed Egr1-/- mice. EGR-1-deficient islets failed to maintain the transcriptional network for β-cell compensatory response. In human pancreatic tissues, EGR1 expression correlated with the expression of β-cell compensatory genes in the non-diabetic group, but not in the diabetic group.
Conclusion: These results suggest that EGR-1 couples the transcriptional network to compensation for the loss of β-cell function and identity. Thus, our study highlights the early stress coupler EGR-1 as a critical factor in the development of pancreatic islet failure.
Keywords: β-cell compensation, β-cell identity, immediate genes, islet failure, stimulus-response coupling
 Global reach, higher impact
Global reach, higher impact