13.3
Impact Factor
Theranostics 2020; 10(10):4374-4382. doi:10.7150/thno.43360 This issue Cite
Review
Advances in CRISPR/Cas-based Gene Therapy in Human Genetic Diseases
1. Key Laboratory of Growth Regulation and Transformation Research of Zhejiang Province, School of Life Sciences, Westlake University, 18 Shilongshan Road, Hangzhou 310024, Zhejiang Province, China.
2. RNA Therapeutics Institute, University of Massachusetts Medical School, Worcester, Massachusetts
3. Program in Molecular Medicine and Department of Molecular, Cell and Cancer Biology, University of Massachusetts Medical School, Worcester, Massachusetts
*Equal contributors
Received 2019-12-24; Accepted 2020-2-25; Published 2020-3-15
Abstract
CRISPR/Cas genome editing is a simple, cost effective, and highly specific technique for introducing genetic variations. In mammalian cells, CRISPR/Cas can facilitate non-homologous end joining, homology- directed repair, and single-base exchanges. Cas9/Cas12a nuclease, dCas9 transcriptional regulators, base editors, PRIME editors and RNA editing tools are widely used in basic research. Currently, a variety of CRISPR/Cas-based therapeutics are being investigated in clinical trials. Among many new findings that have advanced the field, we highlight a few recent advances that are relevant to CRISPR/Cas-based gene therapies for monogenic human genetic diseases.
Keywords: CRISPR/Cas, Gene editing, Gene therapy, Human disease, Genetic disease
Introduction
The past 20 years have witnessed great progress in genome editing techniques, including meganucleases, zinc finger nucleases (ZFN), transcription activator-like effector nucleases (TALENs), and the clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (Cas) nuclease system. These tools hold great potential for treating human disease, especially genetic diseases beyond the reach of traditional approaches [1]. The CRISPR/Cas system has rapidly become the most popular genome editing platform due to its simplicity and adaptability [2-5].
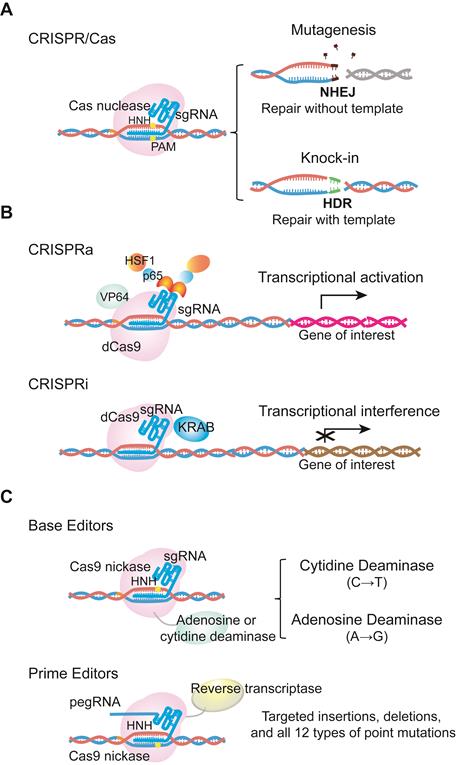
The CRISPR/Cas system was originally discovered as a prokaryotic adaptive immunity system used to recognize and cleave invading nucleic acids [6-8]. Based on this prokaryotic system, scientists have engineered a series of CRISPR/Cas tools for genome editing in mammalian cells, with the list of CRISPR/Cas systems in use continuing to expand. The most commonly used Cas nuclease comes from Streptococcus pyogenes (SpCas9), and belongs to the type II CRISPR system. SpCas9 was the first to be reprogrammed for genome editing in mammalian cells. For specific nucleotide sequence recognition, engineered SpCas9 relies on the guidance of a single-guide RNA (sgRNA). Typically, sgRNA is composed of a scaffold sequence that is bound by the Cas protein, and a custom-designed ∼20 nucleotide spacer that defines the genomic target to be modified. Following hybridization of the spacer to a target genomic sequence that is positioned next to a protospacer adjacent motif (PAM), the target DNA is cleaved, leading to a double-strand break (DSB) [7-9]. The Cas-mediated DSB is subsequently repaired by cellular DNA repair machinery via homology- directed repair (HDR) or the non-homologous end joining (NHEJ) pathway. NHEJ can be used to produce insertions and deletions (indels) that disrupt or inactivate the target gene, while HDR can be used for precise nucleotide sequence modifications, such as point mutation correction [10-12] (Figure 1a).
To date, CRISPR/Cas-based techniques have been applied in various cell types and organisms. For therapeutic genome editing to treat monogenic diseases, CRISPR has the potential to be used directly in patients (in vivo) or in human cells (in vitro). In this review, we focus on CRISPR strategies used to treat human monogenic diseases, and discuss the challenges associated with these approaches.
Examples of CRISPR/Cas9 technological advances. (a) Cas9 is directed by single guide RNA (sgRNA) to the target sequence. Double stranded DNA breaks are subsequently repaired by cellular DNA repair machinery via the NHEJ or HDR pathway. (b) dCas9 fused with transcriptional activators or repressors activates or inhibits the expression of a target gene. These systems are called CRISPRa or CRISPRi. dCas9 indicates catalytically inactive dead Cas9, which is able to bind the target DNA without cutting. CRISPRa, CRISPR activators to activate transcriptional process; CRISPRi, CRISPR inhibitors to interference transcriptional process. (c) Base editors are the combination of Cas9 D10A nickase with cytidine or adenine deaminase to induce G->T or A->G transition. Prime editor, different from base editors, is the fusion protein of Cas9 H840A nickase and reverse transcriptase. It can achieve up to 12 types of base-to-base conversions, and targeted insertions and deletions without DSBs or donor DNA templates. pegRNA, prime editing guide RNA.
Recent advances in CRISPR/Cas technology
Shortly after SpCas9 was applied in mammalian cells, other Cas9 proteins have been studied and developed as genome editing tools. For example, smaller Cas9 proteins derived from Staphylococcus aureus called SaCas9 [13] and Neisseria meningitidis called Nme2Cas9 [14] exhibit gene editing efficiency comparable to that of SpCas9. These smaller Cas9s are more amenable for in vivo delivery than the large SpCas9 (~4.3 kb).
CRISPR/Cas9 technological advances have also enabled various applications of nuclease-deficient Cas9s, which can bind a specific region of the genome without creating DSBs. For example, catalytically inactive dead Cas9 (dCas9) can be fused with various transcription regulatory domains to create CRISPR activators (CRISPRa) or inhibitors (CRISPRi) that activate or silence the expression of a target gene [15] (Figure 1b). dCas9 can also be used as a visualization tool. Chen and colleagues have used dCas9 fused to enhanced green fluorescent protein (EGFP) to visualize repetitive DNA sequences using one sgRNA, or nonrepetitive loci using multiple sgRNAs [16-18]. In addition, David R. Liu's group has fused D10A Cas9 nickase with either cytidine or adenine deaminase to generate cytidine base editors (CBEs) and adenine base editors (ABEs), respectively. CBEs and ABEs generate transitions between A•T and C•G base pairs without causing high levels of double-stranded DNA cleavage in the target genomic region. Importantly, the Liu's group has extended base editing to utilize H840A Cas9 nickase fused with reverse transcriptase to create prime editors (PEs), which can achieve all possible base-to-base conversions (12 in total), as well as targeted insertions and deletions without DSBs or donor DNA templates [19] (Figure 1c).
In addition to DNA editing, Feng Zhang's lab has reported that an RNA-targeting CRISPR system based on Cas13 can target and cleave specific strands of RNA, and subsequently developed strategies called REPAIR (RNA Editing for Programmable A to I Replacement) and RESCUE (RNA Editing for Specific C to U Exchange) to edit RNA [20, 21]. Thus, RNA editing with CRISPR can efficiently modulate target genes at the transcript level in a transient and PAM independent manner. This approach could provide a controllable approach for disease treatment.
Animal diseases models generated by CRISPR listed in this review.
| Corresponding human disease | Targeted gene | Subsrate | Stragegy | Author, year, (Refs) |
|---|---|---|---|---|
| Duchenne muscular dystrophy (DMD) | DMD | Human rhabdomyosarcoma cell line | NHEJ-mediated exon removal | Shimo et al, 2018,(31) |
| Aniridia-related keratopathy (ARK) | PAX6 | Human limbal epithelial cells | NHEJ-mediated mutation | Roux et al, 2018, (32) |
| Osteogenesis Imperfecta (OI) | COL1A1 | Human MCRIi001-A iPSCs line | NHEJ-mediated a single base insertion | Far et al, 2019, (33) |
| X-linked adrenoleukodystrophy (X-ALD) | ABCD1 & ABCD2 | Murine BV-2 immortalized cell line | NHEJ-mediated gene deletion | Raas et al, 2019, (34) |
| Alzheimer's disease | APPS & PSEN1M1 | Human and Mouse IPS Cell line | HDR-mediated mutation | Paquet et al, 2016, (35) |
| Duchenne muscular dystrophy (DMD) | DMD | Mouse | NHEJ-mediated exon removal | Egorova et al, 2019, (36) |
| Atherosclerosis | LDLR | Mouse liver | NHEJ-mediated gene deletion | Jarrett et al, 2018, (37) |
| Obesity (ob/ob) and diabetes (db/db) | LEP & LEPR | Mouse | NHEJ-mediated gene deletion | Roh et al, 2018, (38) |
| Resistance to thyroid hormone due to THRA mutation (RTHα) | THRA | Mouse | HDR-mediated mutation | Markossian et al, 2017, (39) |
| Alzheimer's disease (AD) and frontotemporal dementia (FTD) | MAPT | Mouse | NHEJ-mediated exon removal | Tan et al, 2018, (40) |
| Ryanodine receptor type I (RYR1)-related myopathies (RYR1 RM) | RYR1 | Mouse muscle | HDR-mediated mutation | Brennan et al, 2019, (41) |
| Cystic fibrosis (CF) | CFTR | Sheep | NHEJ-mediated gene deletion | Fan et al, 2018, (42) |
| Diabetes mellitus (DM) | PAX4 | Rabbit | NHEJ-mediated gene deletion | Xu et al, 2018, (43) |
| Huntington's disease (HD) | HTT | Pig | HDR-mediated exon fragments insertion | Yan et al, 2018, (44) |
| Autosomal recessive juvenile parkinsonism | PINK1 | Monkey | NHEJ-mediated gene deletion | Yang et al, 2019, (45) |
Applications of CRISPR in genetic diseases
To date, CRISPR/Cas systems have been used to investigate target genes in genome modification [22], splicing [23], transcription [24] and epigenetic regulation [25], and have been applied in a research setting to investigate and treat genetic diseases [26], infectious diseases [27], cancers [28], and immunological diseases [29, 30]. Among the exciting advances, translational use of CRISPR/Cas in monogenic human genetic diseases has the potential to provide long-term therapy after a single treatment. In this section, we summarize the recent applications of the CRISPR/Cas system in the generation of disease models and in the treatment of genetic diseases in vitro and in vivo.
Disease modeling using CRISPR/Cas
The generation of disease models is necessary for understanding disease mechanisms and developing new therapeutic strategies. CRISPR/Cas has been widely used for creating disease-related cellular models, such as DMD [31], aniridia-related keratopathy (ARK) [32], brittle bone [33], X-linked adrenoleukodystrophy (X-ALD) [34], and Alzheimer's disease [35]. Moreover, researchers have created a series of mouse models using CRISPR/Cas that recapitulate DMD [36], atherosclerosis [37], obesity and diabetes [38], RTHα [39], and Alzheimer's disease [40] (Table 1). One example is the development of a mouse model for ryanodine receptor type I-related myopathies (RYR1 RM), which harbors a patient- relevant point mutation (T4706M) engineered into one allele, and a 16-base pair frameshift deletion engineered into the second allele of the RYR1 gene. Subsequent experiments demonstrated that this mouse model of RYR1 RM is a powerful tool for understanding the pathogenesis of recessive RYR1 RM, and for preclinical testing of therapeutic efficacy [41]. CRISPR/Cas has also been used to generate disease models in large animals, including sheep [42], rabbit [43], pig [44], and monkey [45]. For example, a monkey model was developed to study Parkinson's disease by introducing a PINK1 deletion and revealed a requirement for functional PINK1 in the developing primate brain [45]. CRISPR/Cas technology offers a flexible and user-friendly means of developing disease models to explore the genetic causes of diseases and evaluate therapeutic strategies.
Disease correction using CRISPR/Cas in model organisms and clinical trials
Monogenic diseases affect a large population of patients. In the ClinVar database, more than 75,000 pathogenic genetic variants have been identified [19, 46]. Here we summarize recent therapeutic applications of CRISPR/Cas in model organisms and in clinical trials (Table 2 and Table 3).
Hemoglobinopathies
Inherited blood disorders are good candidates for gene therapies because gene therapy can modify the causative gene in autologous hematopoietic stem cells (HSCs) and correct the hematopoietic system. β-thalassemia and sickle cell disease are two genetic blood diseases. β-thalassemia is due to various mutations including small insertions, single point mutations or deletions in β-globin gene, resulting in loss or reduced β-globin synthesis [47]. Sickle cell disease is caused by a Glu->Val mutation in β-globin subunit of hemoglobin [48, 49], leading to abnormal hemoglobin S. Re-expressing the paralogous γ-globin genes is a universal strategy to ameliorate both β-globin disorders. The Bauer group applied CRISPR/Cas-based cleavage of the GATA1 binding site of the erythroid enhancer. This approach decreases erythroid expression of the γ-globin repressor BCL11A and in turn increases γ-globin expression. This strategy is therapeutically practicable to produce durable fetal hemoglobin induction [50-52] (Table 2).
To date, three clinical trials aiming to treat patients with β-thalassemia and severe sickle cell disease by transfusion of CRIPSR/Cas9 edited CD34+ human HSCs (CTX001) have been initiated by CRISPR Therapeutics in 2018 and Allife Medical Science and Technology Co., Ltd in 2019 (Table 3).
Preclinical CRISPR Therapy in disease models listed in this review.
| Diseases | Target (Gene accession number) | Animal model or substrate | Delivery System | Strategy | Outcome | Author, year, (Refs) |
|---|---|---|---|---|---|---|
| β-thalassemia | HBB (NC_000011.10) | CD34+ HSPCs of β-thalassemia patients | RNP; electroporation | NHEJ-mediated mRNA splicing | 93.0% indel frequency (SpCas9) | Xu et al, 2019 (50) |
| Hemoglobinopathies | BCL11A erythroidenhancer (NC_000002.12) | CD34+ HSPCs from sickle cell disease patient | RNP; electroporation | NHEJ-mediated enhancer disruption | 54.6% reduction of BCL11A expression | Wu et al, 2019, (52) |
| Leber congenital amaurosis type 10 | CEP290 (NC_000012.12 ) | HuCEP290 IVS26 KI mouse eye | AAV; subretinal injection | NHEJ-mediated aberrant splicing | ~ 60% editing rates in mice | Maeder et al, 2019, (53) |
| Duchenne muscular dystrophy (DMD) | Dmd (NC_000086.7) | mdx mice muscle | AAV; intramuscular injection (IM), retro- orbital injection (RO) and intraperitoneal injection (IP) | NHEJ-mediated mutant exon 23 skipping | ~52% of WT (IP) , ~71% of WT (RO), and ~70% of WT (IM) Dystrophin protein levels | Long et al, 2016, (55) |
| Duchenne muscular dystrophy (DMD) | Dmd (NC_000086.7) | mdx mice muscle | AAV; intramuscular injection | NHEJ-mediated mutant exon 23 skipping | ~2% of all alleles from the whole muscle lysate | Nelson et al, 2016, (56) |
| Duchenne muscular dystrophy (DMD) | Dmd (NC_000086.7) | mdx mice muscle | AAV; intraperitoneal injection | NHEJ-mediated mutant exon 23 skipping | 24-47% of total Dmd mRNA in cells including exon23 deletion | Tabebordbar et al, 2016, (57) |
| Congenital muscular dystrophy type 1A (MDC1A) | Lama1 (NC_000083.6 ) | dy2j/dy2j mouse | AAV; intramuscular or tail vein injection | CRISPR activator mediated gene upregulation | 3.6-fold upregulation of Lama1 | Kemaladewi et al, 2019, (60) |
| Hereditary tyrosinemia type I (HTI) | FAH (NC_000073.6) | FAHmut/mut mouse liver | AAV combined with lipid nanoparticles; intravenous injection | HDR-mediated point mutation correction | ~0.8% initial correction rate in total liver DNA; more than 6% FAH+ hepatocytes | Yin et al, 2016, (62) |
| Hereditary tyrosinemia type I (HTI) | FAH (NC_000073.6) | FAHmut/mut mouse hepatocytes | AAV; transplantation | HDR-mediated point mutation correction | 2.6% alleles were correted | VanLith et al, 2019, (63) |
| Hereditary tyrosinaemiatype I (HTI) | FAH (NC_000073.6) | FAHmut/mut mouse liver | plasmids; hydrodynamic tail-vein injection | Adenine base editor mediated point mutation correction | ~0.3% initial correction rate in liver, ~4% FAH+ hepatocytes | Song et al, 2019, (64) |
| α1-antitrypsin deficiency (AATD) | AAT (NC_000078.6 ) | PiZ mouse liver | AAV; intravenous injection | NHEJ-mediated mutant AAT disruption | ~30% idel frequency | Bjursell et al, 2018, (66) |
| α1-antitrypsin deficiency (AATD) | AAT (NC_000078.6 ) | PiZ mouse liver | AAV; intravenous injection | HdR-mediated point mutation correction | ~2% correction rate in liver | Song et al, 2018, (67) |
| Perinatal Lethal Respiratory Failure | SFTPC ( NC_000080.6) | SFTPCI73T; R26mTmG/+ mouse fetus lung | adeno virus; intra-amniotic delivery | NHEJ-mediated mutant SFTPC disruption | ~20% editing in the lung epithelium of fetuses | Alapati et al, 2019, (69) |
| Genetic Deafness | Tmc1 (NC_000085.6) | Beethoven (Bth)mouse ear | AAV; Inner-ear injections | NHEJ-mediated mutant Tmc allele disruption | 2.2% indel frequencies at 55 days after injection; 24% decrease in Bth mRNA | György et al, 2019, (75) |
CRISPR clinical trials for inherited diseases listed in this review.
| Disease | Study title | Strategy | Study phase | Study type | Participants (No., Age) | Company | NCT Number | Website |
|---|---|---|---|---|---|---|---|---|
| Transfusion- Dependent β-thalassemia | A Safety and Efficacy Study Evaluating CTX001 in Subjects With Transfusion-Dependent β-Thalassemia | CTX001 | Phase 1Phase 2 | Interventional | 45 patients, ≥18 and ≤35 years of age | Vertex Pharmaceuticals Incorporated & CRISPR Therapeutics | NCT03655678 | https://clinicaltrials.gov/ct2/show/NCT03655678 |
| Sickle Cell Disease | A Safety and Efficacy Study Evaluating CTX001 in Subjects With Severe Sickle Cell Disease | CTX001 | Phase 1Phase 2 | Interventional | 45 patients, ≥18 and ≤35 years of age | Vertex Pharmaceuticals Incorporated & CRISPR Therapeutics | NCT03745287 | https://clinicaltrials.gov/ct2/show/NCT03745287 |
| β-thalassemia | iHSCs With the Gene Correction of HBB Intervent Subjests With β-thalassemia Mutations | HBB HSC-01 | Early Phase 1 | Interventional | 12 patients, ≥ 2 and ≤ 60 years of age | Allife Medical Science & Technology Co., Ltd. | NCT03728322 | https://clinicaltrials.gov/ct2/show/NCT03728322 |
| Leber congenital amaurosis LCA10 | Single Ascending Dose Study in Participants With LCA10 | AGN-151587 | Phase 1Phase 2 | Interventional | 18 patients, ≥ 3 Years | Allergan & Editas Medicine, Inc. | NCT03872479 | https://clinicaltrials.gov/ct2/show/NCT03872479 |
Data from https://clinicaltrials.gov/
Inherited eye disease
Leber congenital amaurosis (LCA) is a rare genetic eye disease manifesting severe vision loss at birth or infancy. LCA10 caused by mutations in the CEP290 gene is a severe retinal dystrophy. CEP290 gene (~7.5 kb) is too large to be packaged into a single AAV. To overcome this limitation, Editas Medicine developed EDIT-101, a candidate genome editing therapeutic, to correct the CEP290 splicing defect in human cells and in humanized CEP290 mice by subretinal delivery. This approach uses SaCas9 to remove the aberrant splice donor generated by the IVS26 mutation. In the human CEP290 IVS26 knock-in mouse model, over 94% of the treated eyes achieved therapeutic target editing level (10%) when the dose of AAV was not less than 1 × 1012 vg/ml [53]. Allergan and Editas Medicine have initiated a clinical trial of EDIT-101 for the treatment of LCA10 (Table 3).
Autosomal dominant cone-rod dystrophy (CORD6) is induced by a gain-of-function GUCY2D mutation. CRISPR/Cas components delivered by AAV specifically disrupt the early coding sequence of GUCY2D in the photoreceptors of mice and macaques by NHEJ. This study was the first to successfully perform somatic gene editing in primates using AAV-delivered CRISPR/Cas (up to 13% editing efficiency of GUCY2D mutant gene in macaque photoreceptor), and demonstrated the potential of CRISPR/Cas to cure inherited retinal diseases [54].
Muscular genetic disease
DMD, caused by mutations in the dystrophin gene, is the most common form of progressive muscular dystrophy, and is characterized by muscle weakness, loss of ambulation, and premature death. Several groups have used NHEJ to bypass a premature stop codon in exon 23 and restore the expression of dystrophin in neonatal and adult mice after local or systemic delivery of CRISPR/Cas components by AAV [55-57]. Similarly, CRISPR/Cas- induced NHEJ has been used to treat DMD in a DMD dog model after AAV-mediated systemic delivery of CRISPR gene editing components. 3 to 90% of dystrophin was recovered at 8 weeks after systemic delivery in skeletal muscle, the editing efficiency was dependent on muscle type and the muscle histology was improved in treated dogs [58]. In addition, ABE was delivered locally by intramuscular injection of a trans-splicing AAV to cure DMD in a mouse model [59]. These studies highlight the potential application of gene editing for the correction of DMD in patients.
Congenital muscular dystrophy type 1A (MDC1A), one of neuromuscular disorders, usually appears at birth or infancy. It is mainly featured by hypotonia, myasthenia and amyotrophy. MDC1A is caused by loss-of-function mutations in LAMA2, which encodes for laminin-α2. To compensate for the loss of laminin-α2, Ronald D. Cohn and his colleagues used CRISPRa to upregulate LAMA1, which encodes laminin-α1 and is a structurally similar protein to laminin-α2. Upregulation of LAMA1 ameliorates muscle wasting and paralysis in the MDC1A mouse model and provides a novel mutation-independent approach for disease correction [60].
Genetic liver disease
Hereditary tyrosinemia type I (HTI) patients with loss of function FAH mutations accumulate toxic metabolites that cause liver damage. CRISPR/Cas- mediated HDR has been used to correct FAHmut/mut in the HTI mouse model by hydrodynamic injection of plasmids encoding CRISPR/Cas components or by combined delivery of AAV carrying HDR template and sgRNA and of nanoparticles with Cas9 mRNA [61, 62]. VanLith et al. transplanted edited hepatocytes with corrected FAH into recipient FAH-knockout mice and cured HTI mice [63]. Song et al. have used ABE in an adult mouse model of HTI to correct a FAH point mutation [64]. In addition to correcting FAH, several groups have knocked out hydroxyphenylpyruvate dioxygenase (HPD), which acts in the second step of tyrosine catabolism and is an upstream enzyme of FAH, to prevent toxic metabolite accumulation and treat HTI metabolic disease [65].
Patients with alpha-1 antitrypsin deficiency (AATD) develop liver disease due to a toxic gain-of- function mutant allele, as well as progressive lung disease due to the loss of AAT antiprotease function. CRISPR/Cas-mediated NHEJ has been used to disrupt mutant AAT to reduce the pathologic liver phenotype [66], while HDR has been used to correct an AAT point mutation [67].
Congenital genetic lung disease
Congenital genetic lung diseases include cystic fibrosis and inherited surfactant protein (SP) syndromes [68]. Monogenic lung diseases caused by mutations in SP genes of the pulmonary epithelium show perinatal lethal respiratory failure death or chronic diffuse lung disease with few therapeutic options. Using a CRISPR fluorescent reporter system, scientists precisely timed intra-amniotic delivery of CRISPR/Cas9 components into a prenatal mouse model with the human SP gene SFTPCI73T mutation to inactivate mutant SFTPCI73T gene through NHEJ. Prenatal gene editing in SFTPCI73T mutant mice rescued lung pathophysiology, improved lung development, and increased survival rate to 22.8%. For intra- amniotic delivery, the amniotic cavity of embryonic day 16 mouse fetus, in which fetal breathing movements are optimal for fetal lung editing, was injected. After prenatal CRISPR delivery, embryonic day 19 fetus achieved up to 32% SFTPC wild-type airway and alveolar epithelial cells in SFTPCI73T mice, rescued lung pathophysiology by immunohistology, improved lung development by reducing the synthesis of mis trafficked SFTPC mutant proprotein, and increased survival rate to 22.8% [69].
Cystic fibrosis is another life-threatening monogenic lung disease caused by mutations in CFTR gene [70]. Researchers applied CRISPR to precisely corrected CFTR carrying homozygous F508 deletion (F508del) in exon 10 in the induced pluripotent stem cells (iPSC) separated from cystic fibrosis patients [71] and the overall correction efficiency is up to 90% using piggyBac transposase as selection marker. Xu group applied the electroporation of CRISPR/Cas RNP and achieved more than 20% correction rate in patient-derived iPSC cell line with F508del mutation [72]. As expected, CRISPR-induced genetic correction leads to the recovery of CFTR function in airway epithelial cells or proximal lung organoids derived from iPSC.
Genetic deafness
At least half of all cases of profound congenital deafness are caused by genetic mutations and genetically inherited. Approximately 120 deafness- associated genes have been identified, but few treatments are available to slow or reverse genetic deafness [73]. Recently, David R. Liu's group employed cationic lipid-mediated in vivo delivery of Cas9-guide RNA complexes to disrupt the dominant deafness-associated allele in the humanized transmembrane channel-like 1 (Tmc1) Beethoven (Bth) mouse model and ameliorated the hearing loss in these animals [74]. David P. Corey's group screened 14 Cas9/sgRNA combinations and identified that SaCas9-KKH/gRNA could specially and safely recognize mutant Tmc1 but not wildtype allele in vitro and in vivo, which provides a strategy to efficiently and selectively disrupt the dominant single nucleotide mutation rather than the wild-type alleles [75].
Overcoming limitations of CRISPR/Cas-based gene therapy
Extensive work is being done with CRISPR/Cas in disease research and recent reviews had summarized the advantages of CRISPR/Cas [76, 77]. The safety and efficacy of CRISPR/Cas9-based gene therapies need to be evaluated and refined before these therapies are applied in patients [78]. One of the common limitations for CRISPR/Cas is that not all the mutation locus harbors the PAM motif, which the target recognition relies on. Besides, the challenges for using CRISPR/Cas as gene therapy include editing at off-target genomic sites, delivery vehicle, immunogenicity, and DNA damage response.
Off-target effects of CRISPR/Cas
Despite significant advances in understanding the CRISPR/Cas9 system, concerns remain regarding off-target effects. Indeed, several groups found a tradeoff between activity and specificity of CRISPR/ Cas9, identifying off-target DNA cleavage by genome wide deep sequencing technique [79-81]. Moreover, CBEs and ABEs cause transcriptome-wide off-target RNA editing [82, 83]. Thus, unwanted off targets are concerns for the application of CRISPR. However, off-target effects can be reduced with sgRNA selection and optimization. Also, verification of in vivo off-targets (VIVO) can be used for defining and quantifying off-target editing of nucleases in whole organisms [84]. The recently developed anti-CRISPR proteins could conditionally control the activity of the CRISPR system [85-88], which may show the potential in reducing off-target effects. The development of more sensitive methods is necessary for detecting off-target editing at both genome and transcriptome levels.
In vivo delivery of CRISPR/Cas
AAV is the most widely used in vivo delivery of CRISPR/Cas. However, AAV has a limited packaging capacity, hindering all-in-one delivery of CRISPR/ Cas components, in particular larger Cas-derived base editor and prime editor. This has led to continued development of smaller Cas9 orthologues like SaCas9 [13]. For instance, saCas9 or NmeCas9 and sgRNA have been combined into a single AAV vector for inducing indels to correct disease. For disease correction by HDR or base editors, dual AAV or split AAV vectors can be used to circumvent packaging size limitations [89, 90]. A disadvantage of such an approach is the requirement of uptake and expression of both AAV vectors into the same cell at roughly the same time to ensure intracellular Cas9:sgRNA complex formation.
CRISPR/Cas components can also be delivered by non-viral methods, for instance, Cas9 mRNA and sgRNA can be delivered to mouse liver by nanoparticles [62]. But the external and internal barriers for nanoparticles entering the cell and nucleus must be considered. Currently, nanoparticles carrying CRISPR/Cas components are largely applied to mice and delivered into liver. Because the liver contains fenestrated capillary endothelia. Further improvement of nanoparticle-based CRISPR/Cas components delivery systems is needed for other target tissues.
Immune response stimulated by CRISPR/Cas
The application of CRISPR/Cas systems raises concerns over immunogenicity of the bacterially- derived Cas9 protein [91]. In a recent study, Charlesworth et al. demonstrated that anti-Cas9 responses are present in healthy human adults [92]. In 34 human blood samples, anti-Cas9 IgG antibodies were detected against SaCas9 (79% of samples), and against SpCas9 (65% of samples). The immunogenicity of SpCas9 in healthy humans has been reported by Michael's group. Specifically, they found that high prevalence of effector T cells towards SpCas9 exist prior to the delivery of SpCas9 [93]. This issue will need to be addressed in the clinical applications of CRISPR/Cas.
DNA damage response activated by CRISPR/Cas
In CRISPR/Cas gene editing via NHEJ and HDR, DSBs are generated at the target sites. DBS- based repair activates a p53-dependent DNA damage response and induces transient cell cycle arrest, leading to a decrease in efficiency of template- mediated precision genome editing [94]. In human pluripotent stem cells, p53-deficient cells are more susceptible to CRISPR-mediated modification [95]. These findings suggest that, during clinical trials, CRISPR-engineered cells or organs in patients should be monitored for p53 function. To avoid DSB triggered p53-mediated response, base editors (ABE and CBE) and prime editors can be applied for precision gene editing-mediated target gene correction.
Conclusion and perspectives of using CRISPR/Cas in the clinic
CRISPR/Cas has already shown great potential in generating disease models and correcting monogenic disease mutations. The CRISPR disease models can accelerate the discovery and development of drug targets. In addition to the widely used type II CRISPR/Cas systems, continued discovery and development of CRISPR systems from prokaryotic species has generated new technologies. For example, DN1S-SpCas9 fusion protein blocks local NHEJ events and increases HDR frequency [96]. Moreover, Cas13a-based RNA-targeting tools enable RNA changes that are temporally and spatially controllable, and will broaden and facilitate the application of RNA therapy in human diseases. Before the application of CRISPR for human disease correction, efforts are needed to optimize and maximize the editing efficiency as well as minimize off-targets and develop novel tools to specifically deliver the CRISPR components to the target tissue for gene editing [97, 98]. As CRISPR/Cas-based gene therapy enters clinical trials (Table 3), this technology holds great potential for treating genetic diseases particularly for the present incurable ones and enhancing cell therapies.
Abbreviations
ZFN, zinc finger nucleases; TALENS, transcription activator-like effector nucleases; CRISPR, clustered regularly interspaced short palindromic repeats; Cas, CRISPR-associated nuclease; SpCas9, Cas nuclease comes from Streptococcus pyogenes; sgRNA, single-guide RNA; PAM, protospacer adjacent motif; DSB, double-strand break; NHEJ, non- homologous end joining; HDR, homology-directed repair; indels, insertions and deletions; CRISPRa, CRISPR activators; CRISPRi, CRISPR inhibitors; AAV, adeno-associated virus; CBEs, cytidine base editors; ABEs, adenine base editors; PEs, prime editors; DMD, duchenne muscular dystrophy; HTI, hereditary tyrosinemia type I; AAV, adeno-associated virus.
Acknowledgements
The authors thank Craig. Mello, Scot Wolfe, En-Zhi Shen, and Erik. Sontheimer for discussions, Suet-Yan Kwan and Emily Haberlin for editing the manuscript, and Ya-Ping Shen for raw figure preparation. Wen Xue was supported by grants from the National Institutes of Health (DP2HL137167, P01HL131471 and UG3HL147367), American Cancer Society (129056-RSG-16-093), the Lung Cancer Research Foundation, and the Cystic Fibrosis Foundation. Chun-Qing Song was supported by start funding of Westlake University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. High KA, Roncarolo MG. Gene Therapy. N Engl J Med. 2019;381:455-64
2. Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096
3. Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21:121-31
4. Yin H, Xue W, Anderson DG. CRISPR-Cas: a tool for cancer research and therapeutics. Nat Rev Clin Oncol. 2019;16:281-95
5. Fellmann C, Gowen BG, Lin PC, Doudna JA, Corn JE. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov. 2017;16:89-100
6. Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174-82
7. Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109:E2579-86
8. Marraffini LA, Sontheimer EJ. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet. 2010;11:181-90
9. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N. et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819-23
10. Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397-405
11. Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181-211
12. Chiruvella KK, Liang Z, Wilson TE. Repair of double-strand breaks by end joining. Cold Spring Harb Perspect Biol. 2013;5:a012757
13. Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186-91
14. Edraki A, Mir A, Ibraheim R, Gainetdinov I, Yoon Y, Song CQ. et al. A Compact, High-Accuracy Cas9 with a Dinucleotide PAM for In Vivo Genome Editing. Mol Cell. 2019;73:714-26 e4
15. Kampmann M. CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem Biol. 2018;13:406-16
16. Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479-91
17. Wu X, Mao S, Ying Y, Krueger CJ, Chen AK. Progress and Challenges for Live-cell Imaging of Genomic Loci Using CRISPR-based Platforms. Genomics Proteomics Bioinformatics. 2019;17:119-28
18. Chen B, Zou W, Xu H, Liang Y, Huang B. Efficient labeling and imaging of protein-coding genes in living cells using CRISPR-Tag. Nat Commun. 2018;9:5065
19. Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149-57
20. Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J. et al. RNA editing with CRISPR-Cas13. Science. 2017;358:1019-27
21. Abudayyeh OO, Gootenberg JS, Franklin B, Koob J, Kellner MJ, Ladha A. et al. A cytosine deaminase for programmable single-base RNA editing. Science. 2019;365:382
22. Oakes BL, Fellmann C, Rishi H, Taylor KL, Ren SM, Nadler DC. et al. CRISPR-Cas9 Circular Permutants as Programmable Scaffolds for Genome Modification. Cell. 2019;176:254-67 e16
23. Yuan J, Ma Y, Huang T, Chen Y, Peng Y, Li B. et al. Genetic Modulation of RNA Splicing with a CRISPR-Guided Cytidine Deaminase. Mol Cell. 2018;72:380-94 e7
24. Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ. et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280-4
25. Xie N, Zhou Y, Sun Q, Tang B. Novel Epigenetic Techniques Provided by the CRISPR/Cas9 System. Stem Cells Int. 2018;2018:7834175
26. Papasavva P, Kleanthous M, Lederer CW. Rare Opportunities: CRISPR/Cas-Based Therapy Development for Rare Genetic Diseases. Mol Diagn Ther. 2019;23:201-22
27. Kennedy EM, Cullen BR. Gene Editing: A New Tool for Viral Disease. Annu Rev Med. 2017;68:401-11
28. Huang CH, Lee KC, Doudna JA. Applications of CRISPR-Cas Enzymes in Cancer Therapeutics and Detection. Trends Cancer. 2018;4:499-512
29. Xiong X, Chen M, Lim WA, Zhao D, Qi LS. CRISPR/Cas9 for Human Genome Engineering and Disease Research. Annu Rev Genomics Hum Genet. 2016;17:131-54
30. Ferdosi SR, Ewaisha R, Moghadam F, Krishna S, Park JG, Ebrahimkhani MR. et al. Multifunctional CRISPR-Cas9 with engineered immunosilenced human T cell epitopes. Nat Commun. 2019;10:1842
31. Shimo T, Hosoki K, Nakatsuji Y, Yokota T, Obika S. A novel human muscle cell model of Duchenne muscular dystrophy created by CRISPR/Cas9 and evaluation of antisense-mediated exon skipping. J Hum Genet. 2018;63:365-75
32. Roux LN, Petit I, Domart R, Concordet JP, Qu J, Zhou H. et al. Modeling of Aniridia-Related Keratopathy by CRISPR/Cas9 Genome Editing of Human Limbal Epithelial Cells and Rescue by Recombinant PAX6 Protein. Stem Cells. 2018;36:1421-9
33. Hosseini Far H, Patria YN, Motazedian A, Elefanty AG, Stanley EG, Lamande SR. et al. Generation of a heterozygous COL1A1 (c.3969_3970insT) osteogenesis imperfecta mutation human iPSC line, MCRIi001-A-1, using CRISPR/Cas9 editing. Stem Cell Res. 2019;37:101449
34. Raas Q, Gondcaille C, Hamon Y, Leoni V, Caccia C, Menetrier F. et al. CRISPR/Cas9-mediated knockout of Abcd1 and Abcd2 genes in BV-2 cells: novel microglial models for X-linked Adrenoleukodystrophy. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:704-14
35. Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S. et al. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. 2016;533:125-9
36. Egorova TV, Zotova ED, Reshetov DA, Polikarpova AV, Vassilieva SG, Vlodavets DV. et al. CRISPR/Cas9-generated mouse model of Duchenne muscular dystrophy recapitulating a newly identified large 430 kb deletion in the human DMD gene. Dis Model Mech. 2019:12
37. Jarrett KE, Lee C, De Giorgi M, Hurley A, Gillard BK, Doerfler AM. et al. Somatic Editing of Ldlr With Adeno-Associated Viral-CRISPR Is an Efficient Tool for Atherosclerosis Research. Arterioscler Thromb Vasc Biol. 2018;38:1997-2006
38. Roh JI, Lee J, Park SU, Kang YS, Lee J, Oh AR. et al. CRISPR-Cas9-mediated generation of obese and diabetic mouse models. Exp Anim. 2018;67:229-37
39. Markossian S, Guyot R, Richard S, Teixeira M, Aguilera N, Bouchet M. et al. CRISPR/Cas9 Editing of the Mouse Thra Gene Produces Models with Variable Resistance to Thyroid Hormone. Thyroid. 2018;28:139-50
40. Tan DCS, Yao S, Ittner A, Bertz J, Ke YD, Ittner LM. et al. Generation of a New Tau Knockout (tauDeltaex1) Line Using CRISPR/Cas9 Genome Editing in Mice. J Alzheimers Dis. 2018;62:571-8
41. Brennan S, Garcia-Castaneda M, Michelucci A, Sabha N, Malik S, Groom L. et al. Mouse model of severe recessive RYR1-related myopathy. Hum Mol Genet. 2019;28:3024-36
42. Fan Z, Perisse IV, Cotton CU, Regouski M, Meng Q, Domb C. et al. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI Insight. 2018;3:e123529
43. Xu Y, Wang Y, Song Y, Deng J, Chen M, Ouyang H. et al. Generation and Phenotype Identification of PAX4 Gene Knockout Rabbit by CRISPR/Cas9 System. G3 (Bethesda). 2018;8:2833-40
44. Yan S, Tu Z, Liu Z, Fan N, Yang H, Yang S. et al. A Huntingtin Knockin Pig Model Recapitulates Features of Selective Neurodegeneration in Huntington's Disease. Cell. 2018;173:989-1002 e13
45. Yang W, Li S, Li XJ. A CRISPR monkey model unravels a unique function of PINK1 in primate brains. Mol Neurodegener. 2019;14:17
46. Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S. et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44:D862-8
47. Thein SL. The molecular basis of beta-thalassemia. Cold Spring Harb Perspect Med. 2013;3:a011700
48. Heywood JD, Karon M, Weissman S. Amino Acids: Incorporation into α- and β-Chains of Hemoglobin by Normal and Thalassemic Reticulocytes. Science. 1964;146:530
49. Telen MJ, Malik P, Vercellotti GM. Therapeutic strategies for sickle cell disease: towards a multi-agent approach. Nat Rev Drug Discov. 2019;18:139-58
50. Xu S, Luk K, Yao Q, Shen AH, Zeng J, Wu Y. et al. Editing aberrant splice sites efficiently restores β-globin expression in β-thalassemia. Blood. 2019;133:2255-62
51. Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, Shalem O. et al. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 2015;527:192-7
52. Wu Y, Zeng J, Roscoe BP, Liu P, Yao Q, Lazzarotto CR. et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat Med. 2019;25:776-83
53. Maeder ML, Stefanidakis M, Wilson CJ, Baral R, Barrera LA, Bounoutas GS. et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med. 2019;25:229-33
54. McCullough KT, Boye SL, Fajardo D, Calabro K, Peterson JJ, Strang CE. et al. Somatic Gene Editing of GUCY2D by AAV-CRISPR/Cas9 Alters Retinal Structure and Function in Mouse and Macaque. Hum Gene Ther. 2019;30:571-89
55. Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E. et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351:400-3
56. Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM. et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351:403-7
57. Tabebordbar M, Zhu K, Cheng JKW, Chew WL, Widrick JJ, Yan WX. et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351:407-11
58. Amoasii L, Hildyard JCW, Li H, Sanchez-Ortiz E, Mireault A, Caballero D. et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science. 2018;362:86-91
59. Ryu SM, Koo T, Kim K, Lim K, Baek G, Kim ST. et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat Biotechnol. 2018;36:536-9
60. Kemaladewi DU, Bassi PS, Erwood S, Al-Basha D, Gawlik KI, Lindsay K. et al. A mutation-independent approach for muscular dystrophy via upregulation of a modifier gene. Nature. 2019;572:125-30
61. Yin H, Xue W, Chen S, Bogorad RL, Benedetti E, Grompe M. et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32:551-3
62. Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q. et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34:328-33
63. VanLith CJ, Guthman RM, Nicolas CT, Allen KL, Liu Y, Chilton JA. et al. Ex Vivo Hepatocyte Reprograming Promotes Homology-Directed DNA Repair to Correct Metabolic Disease in Mice After Transplantation. Hepatol Commun. 2019;3:558-73
64. Song C-Q, Jiang T, Richter M, Rhym LH, Koblan LW, Zafra MP. et al. Adenine base editing in an adult mouse model of tyrosinaemia. Nat Biomed Eng. 2020;4:125-30
65. Pankowicz FP, Barzi M, Legras X, Hubert L, Mi T, Tomolonis JA. et al. Reprogramming metabolic pathways in vivo with CRISPR/Cas9 genome editing to treat hereditary tyrosinaemia. Nat Commun. 2016;7:12642
66. Bjursell M, Porritt MJ, Ericson E, Taheri-Ghahfarokhi A, Clausen M, Magnusson L. et al. Therapeutic Genome Editing With CRISPR/Cas9 in a Humanized Mouse Model Ameliorates alpha1-antitrypsin Deficiency Phenotype. EBioMedicine. 2018;29:104-11
67. Song CQ, Wang D, Jiang T, O'Connor K, Tang Q, Cai L. et al. In Vivo Genome Editing Partially Restores Alpha1-Antitrypsin in a Murine Model of AAT Deficiency. Hum Gene Ther. 2018;29:853-60
68. Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med. 2010;61:105-19
69. Alapati D, Zacharias WJ, Hartman HA, Rossidis AC, Stratigis JD, Ahn NJ. et al. In utero gene editing for monogenic lung disease. Sci Transl Med. 2019:11
70. Rogan MP, Stoltz DA, Hornick DB. Cystic Fibrosis Transmembrane Conductance Regulator Intracellular Processing, Trafficking, and Opportunities for Mutation-Specific Treatment. CHEST. 2011;139:1480-90
71. Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E. et al. Functional Gene Correction for Cystic Fibrosis in Lung Epithelial Cells Generated from Patient iPSCs. Cell Rep. 2015;12:1385-90
72. Ruan J, Hirai H, Yang D, Ma L, Hou X, Jiang H. et al. Efficient Gene Editing at Major CFTR Mutation Loci. Mol Ther Nucleic Acids. 2019;16:73-81
73. Muller U, Barr-Gillespie PG. New treatment options for hearing loss. Nat Rev Drug Discov. 2015;14:346-65
74. Gao X, Tao Y, Lamas V, Huang M, Yeh WH, Pan B. et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553:217-21
75. Gyorgy B, Nist-Lund C, Pan B, Asai Y, Karavitaki KD, Kleinstiver BP. et al. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat Med. 2019;25:1123-30
76. Molla KA, Yang Y. CRISPR/Cas-Mediated Base Editing: Technical Considerations and Practical Applications. Trends Biotechnol. 2019;37:1121-42
77. Knott GJ, Doudna JA. CRISPR-Cas guides the future of genetic engineering. Science. 2018;361:866
78. Rich K, Terry SF. CRISPR-Cas9: New Heights, New Hesitations. Genet Test Mol Biomarkers. 2018;22:635-6
79. Park SH, Lee CM, Dever DP, Davis TH, Camarena J, Srifa W. et al. Highly efficient editing of the beta-globin gene in patient-derived hematopoietic stem and progenitor cells to treat sickle cell disease. Nucleic Acids Res. 2019;47:7955-72
80. Wang D, Zhang C, Wang B, Li B, Wang Q, Liu D. et al. Optimized CRISPR guide RNA design for two high-fidelity Cas9 variants by deep learning. Nat Commun. 2019;10:4284
81. Ikeda A, Fujii W, Sugiura K, Naito K. High-fidelity endonuclease variant HypaCas9 facilitates accurate allele-specific gene modification in mouse zygotes. Commun Biol. 2019;2:371
82. Grunewald J, Zhou R, Garcia SP, Iyer S, Lareau CA, Aryee MJ. et al. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature. 2019;569:433-7
83. Zhou C, Sun Y, Yan R, Liu Y, Zuo E, Gu C. et al. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature. 2019;571:275-8
84. Akcakaya P, Bobbin ML, Guo JA, Malagon-Lopez J, Clement K, Garcia SP. et al. In vivo CRISPR editing with no detectable genome-wide off-target mutations. Nature. 2018;561:416-9
85. Nakamura M, Srinivasan P, Chavez M, Carter MA, Dominguez AA, La Russa M. et al. Anti-CRISPR-mediated control of gene editing and synthetic circuits in eukaryotic cells. Nat Commun. 2019;10:194
86. Zhang F, Song G, Tian Y. Anti-CRISPRs: The natural inhibitors for CRISPR-Cas systems. Animal Model Exp Med. 2019;2:69-75
87. Rauch BJ, Silvis MR, Hultquist JF, Waters CS, McGregor MJ, Krogan NJ. et al. Inhibition of CRISPR-Cas9 with Bacteriophage Proteins. Cell. 2017;168:150-8.e10
88. Pawluk A, Amrani N, Zhang Y, Garcia B, Hidalgo-Reyes Y, Lee J. et al. Naturally Occurring Off-Switches for CRISPR-Cas9. Cell. 2016;167:1829-38.e9
89. Hirsch ML, Agbandje-McKenna M, Samulski RJ. Little vector, big gene transduction: fragmented genome reassembly of adeno-associated virus. Mol Ther. 2010;18:6-8
90. Hirsch ML, Wolf SJ, Samulski RJ. Delivering Transgenic DNA Exceeding the Carrying Capacity of AAV Vectors. Methods Mol Biol. 2016;1382:21-39
91. Wang D, Mou H, Li S, Li Y, Hough S, Tran K. et al. Adenovirus-Mediated Somatic Genome Editing of Pten by CRISPR/Cas9 in Mouse Liver in Spite of Cas9-Specific Immune Responses. Hum Gene Ther. 2015;26:432-42
92. Charlesworth CT, Deshpande PS, Dever DP, Camarena J, Lemgart VT, Cromer MK. et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 2019;25:249-54
93. Wagner DL, Amini L, Wendering DJ, Burkhardt LM, Akyuz L, Reinke P. et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat Med. 2019;25:242-8
94. Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24:927-30
95. Ihry RJ, Worringer KA, Salick MR, Frias E, Ho D, Theriault K. et al. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat Med. 2018;24:939-46
96. Jayavaradhan R, Pillis DM, Goodman M, Zhang F, Zhang Y, Andreassen PR. et al. CRISPR-Cas9 fusion to dominant-negative 53BP1 enhances HDR and inhibits NHEJ specifically at Cas9 target sites. Nat Commun. 2019;10:2866
97. Ledford H. CRISPR babies: when will the world be ready? Nature. 2019;570:293-6
98. Chaterji S, Ahn EH, Kim D-H. CRISPR Genome Engineering for Human Pluripotent Stem Cell Research. Theranostics. 2017;7:4445-69
Author contact
![]() Corresponding authors: Wen.Xueedu; songchunqingedu.cn
Corresponding authors: Wen.Xueedu; songchunqingedu.cn
 Global reach, higher impact
Global reach, higher impact