13.3
Impact Factor
Theranostics 2020; 10(10):4453-4465. doi:10.7150/thno.42354 This issue Cite
Research Paper
PACAP neuropeptide promotes Hepatocellular Protection via CREB-KLF4 dependent autophagy in mouse liver Ischemia Reperfusion Injury
1. Department of Surgery, Division of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital, Zhejiang University, School of Medicine, Hangzhou, Zhejiang, China.
2. Dumont-UCLA Transplant Center, Division of Liver and Pancreas Transplantation, Department of Surgery, David Geffen School of Medicine at University of California-Los Angeles, Los Angeles, CA, USA.
*These authors contributed equally to this work.
Abstract
Organ ischemia reperfusion injury (IRI), associated with acute hepatocyte death, remains an unresolved problem in clinical orthotopic liver transplantation (OLT). Autophagy, an intracellular self-digesting progress, is responsible for cell reprograming required to regain post-stress homeostasis.
Methods: Here, we analyzed the cytoprotective mechanism of pituitary adenylate cyclase-activating polypeptide (PACAP)-promoted hepatocellular autophagy in a clinically relevant mouse model of extended hepatic cold storage (4 °C UW solution for 20 h) followed by syngeneic OLT.
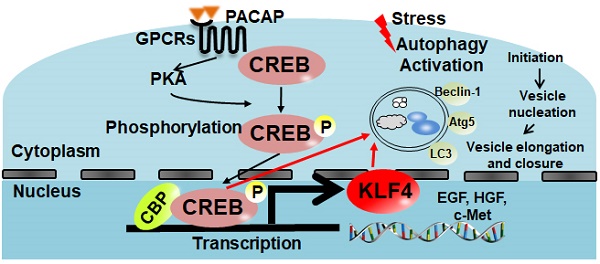
Results: In contrast to 41.7% of liver graft failure by day 7 post-transplant in control group, PACAP treatment significantly improved graft survival (91.7% by day 14), and promoted autophagy-associated regeneration programs in OLT. In parallel in vitro studies, PACAP-enhanced autophagy ameliorated cellular damage (LDH/ALT levels), and diminished necrosis in H2O2-stressed primary hepatocytes. Interestingly, PACAP not only induced nuclear cAMP response element-binding protein (CREB), but also triggered reprogramming factor Kruppel-like factor 4 (KLF4) expression in IR-stressed OLT. Indeed, CREB inhibition attenuated hepatic autophagy and recreated hepatocellular injury in otherwise PACAP-protected livers. Furthermore, CREB inhibition suppressed PACAP-induced KLF4 expression, whereas KLF4 blockade abolished PACAP-promoted autophagy and neutralized PACAP-mediated hepatoprotection both in vivo and in vitro.
Conclusion: Current study documents the essential neural regulation of PACAP-promoted autophagy in hepatocellular homeostasis in OLT, which provides the emerging therapeutic principle to combat hepatic IRI in OLT.
Keywords: Liver Ischemia Reperfusion Injury, Orthotopic Liver Transplantation, PACAP, KLF4, CREB
 Global reach, higher impact
Global reach, higher impact