13.3
Impact Factor
Theranostics 2020; 10(12):5195-5208. doi:10.7150/thno.45017 This issue Cite
Research Paper
Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy
1. Department of Hepatobiliary and Pancreatic Surgery, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310009
2. MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China
3. Key Laboratory of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Tumor of Zhejiang Province, Hangzhou, Zhejiang 310009
4. Research Center of Diagnosis and Treatment Technology for Hepatocellular Carcinoma of Zhejiang Province, Clinical Research Center of Hepatobiliary and Pancreatic Diseases of Zhejiang Province, Hangzhou, Zhejiang 310009
5. Clinical Medicine Innovation Center of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Disease of Zhejiang University, Hangzhou, Zhejiang
6. Clinical Research Center of Hepatobiliary and Pancreatic Diseases of Zhejiang Province, Hangzhou, Zhejiang 310009
7. State Key Laboratory of Crystal Materials, Shandong University, Jinan 250100
8. Department of Hepatobiliary and Pancreatic Surgery, the First Hospital of Jiaxing, Jiaxing, Zhejiang 330440
# These authors contribute equally.
Abstract
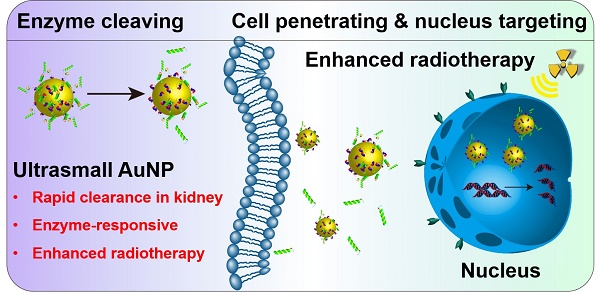
Two important features are required for promising radiosensitizers: one is selective tumor cell targeting to enhance the therapeutic outcome via lethal DNA damage and the other is rapid clearance to enable excellent biocompatibility for potential clinical application. Herein, ultrasmall gold nanoparticles (Au NPs) with diameter smaller than 5 nm were prepared and covered with a multifunctional peptide to endow them with selective tumor cell uptake capability. Combined with X-ray irradiation, the responsive Au NPs demonstrated superior radio-sensitizing toxicity and rapid renal clearance in vivo.
Methods: A responsive peptide (Tat-R-EK) consists of three build blocks were used: a cell and even nuclear penetrating block derived from human immunodeficiency virus-1 transactivator of transcription protein (Tat), an cathepsin B cleavable linker, and a zwitterionic antifouling block. Ultrasmall Au NPs were prepared and then covered by the peptide via the Au-S bonds between gold and thiol groups from cysteine. The morphology, colloidal stability and the responsiveness of obtained Au@Tat-R-EK NPs were studied using transmittance electron microscopy and dynamic laser scattering. The selective cancer cell uptake and accumulation of Au@Tat-R-EK NPs in cancer tissue were studied via ICP-MS in vitro and in vivo, respectively. The cytotoxicity of Au@Tat-R-EK NPs on HepG2 cancer cells was evaluated in terms of cell viability, DNA damage, intracellular reactive oxygen species generation, and apoptosis analysis. Finally, the biocompatibility and tumor destruction ability against orthotopic LM3 liver cancers were verified in vivo.
Results: Multifunctional peptide modified ultrasmall Au NPs were successfully prepared. The Au NPs exhibited enough colloidal stability and cathepsin B-responsive surface change, leading to selectively uptake by cancer cells in vitro and accumulation to tumor sites in vivo. Combined with X-ray irradiation, the responsive Au NPs demonstrated superior radio-sensitizing cytotoxicity in vitro and therapeutic outcome on mouse liver cancer in vivo. The ultrasmall size enables rapid clearance of the Au NPs, guarantees the biocompatibility in vivo for potential clinical applications.
Conclusion: Some obstacles faced by the Au NPs-based radiotherapy, such as short circulation half-life, non-specific distribution, slow clearance and low radio-sensitizing effect, were effective solved through rational design of the smart nanomedicine. This work provides new insight in designing tumor microenvironment-responsive nanomedicine in cancer radiotherapy.
Keywords: enzyme-responsive, Tat peptide, zwitterionic peptide, ultrasmall gold nanoparticles, cancer radiotherapy
 Global reach, higher impact
Global reach, higher impact