13.3
Impact Factor
Theranostics 2020; 10(12):5357-5367. doi:10.7150/thno.42224 This issue Cite
Research Paper
C-Met targeted fluorescence molecular endoscopy in Barrett's esophagus patients and identification of outcome parameters for phase-I studies
1. Department of Gastroenterology and Hepatology, University Medical Center Groningen, Groningen, The Netherlands;
2. Department of Oral and Maxillofacial Surgery, University Medical Center Groningen, Groningen, The Netherlands;
3. Department of Pathology, University Medical Center Groningen, Groningen, The Netherlands;
4. Department of Surgery and Medical Imaging Center, University Medical Center Groningen, The Netherlands;
5. TRACER EUROPE B.V. / AxelaRx, Groningen, The Netherlands;
6. Center for Optical Diagnostics and Therapy, Department of Otorhinolaryngology and Head and Neck Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
*Both authors share first authorship
Abstract
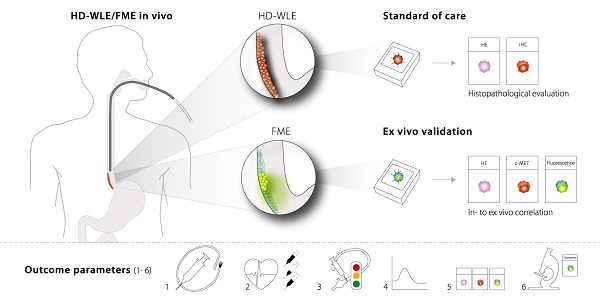
Fluorescence molecular endoscopy (FME) is an emerging technique in the field of gastroenterology that holds potential to improve diagnosis and guide therapy, by serving as a 'red-flag' endoscopic imaging technique. Here, we investigated the safety, feasibility and optimal method of administration of EMI-137, targeting c-Met, during FME in Barrett's Esophagus (BE) and report several outcome parameters for early phase FME studies.
Methods: FME was performed in 15 Barrett's neoplasia patients. EMI-137 was administered to three cohorts of five patients: 0.13 mg/kg intravenously (IV); 0.09 mg/kg IV or topically at a dose of 200 μg/cm BE (n=1) or 100 μg/cm BE (n=4). Fluorescence was visualized in vivo, quantified in vivo using multi-diameter single-fiber reflectance, single-fiber fluorescence (MDSFR/SFF) spectroscopy and correlated to histopathology and immunohistochemistry. EMI-137 localization was assessed using fluorescence microscopy.
Results: FME using different IV and topical doses of EMI-137 appeared to be safe and correctly identified 16/18 lesions, although modest target-to-background ratios were observed (median range of 1.12-1.50). C-Met overexpression varied between lesions, while physiological expression in the stomach-type epithelium was observed. Microscopically, EMI-137 accumulated around the neoplastic cell membranes. We identified several outcome parameters important for the validation of EMI-137 for FME: 1) the optimal administration route; 2) optimal dose and safety; 3) in vivo FME contrast; 4) quantification of intrinsic fluorescence; 5) ex vivo correlation of fluorescence, histopathology and target expression; and 6) microscopic tracer distribution.
Conclusions: C-Met targeted FME using EMI-137 may not be the ideal combination to improve BE surveillance endoscopies, however the identified outcome parameters may serve as a valuable guidance for designing and performing future early phase clinical FME studies, independent of which fluorescent tracer is investigated.
Keywords: Structured roadmap, standardized fluorescence molecular endoscopy methodology, Barrett's esophagus, EMI-137 targeting c-Met.
 Global reach, higher impact
Global reach, higher impact