13.3
Impact Factor
Theranostics 2020; 10(12):5412-5434. doi:10.7150/thno.45214 This issue Cite
Research Paper
Identification of a novel microRNA-141-3p/Forkhead box C1/β-catenin axis associated with rheumatoid arthritis synovial fibroblast function in vivo and in vitro
1. Department of Orthopaedics, The First Affiliated Hospital of Anhui Medical University, Anhui, China
2. Department of Plastic Surgery, The Fourth Affiliated Hospital of Anhui Medical University, Anhui, China
3. Department of Orthopaedics, The Fourth Affiliated Hospital of Anhui Medical University, Anhui, China
4. Department of Radiology, The First Affiliated Hospital of Anhui Medical University, Anhui, China
5. Department of Pathology, The Fourth Affiliated Hospital of Anhui Medical University, Anhui, China
Abstract
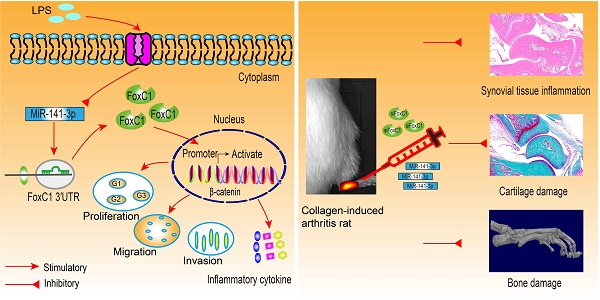
Rationale: Rheumatoid arthritis (RA) is a prototype of inflammatory arthritis in which synovial fibroblasts (SFs) play key roles in cartilage and bone destruction through tumor-like proliferation, migration, invasion and inflammation. This study aimed to research forkhead box protein C1 (FoxC1) and microRNA (miR)-141-3p, which modulate pathological changes in the synovial membrane, to find possible strategies for treating RA.
Methods: FoxC1, β-catenin and miR-141-3p gene expression in synovial tissues and SFs was quantified by real-time PCR; FoxC1 and β-catenin protein levels were evaluated by immunohistochemistry, immunofluorescence, and Western blotting. We transiently transfected human SFs with FoxC1 and β-catenin overexpression and silencing vectors and assessed proliferation, migration, invasion and inflammation by cell function and enzyme-linked immunosorbent assays. We also assessed downstream signaling activation using immunofluorescence, real-time PCR and Western blotting. Double luciferase, coimmunoprecipitation and chromatin immunoprecipitation assays were used to verify miR-141-3p, FoxC1 and β-catenin gene and protein combinations. Finally, the therapeutic effects of FoxC1 silencing and miR-141-3p overexpression were evaluated in type II collagen-induced arthritis (CIA) rats.
Results: We found that FoxC1 expression was significantly upregulated in synovium and SFs in both RA patients and rats with collagen-induced arthritis (CIA). FoxC1 overexpression increased β-catenin messenger RNA (mRNA) and protein levels and upregulated cyclin D1, c-Myc, fibronectin and matrix metalloproteinase 3 (MMP3) mRNA and protein expression in RA SFs (RASFs). In contrast, FoxC1 knockdown reduced β-catenin mRNA and protein levels as well as cyclin D1, c-Myc, and fibronectin mRNA and protein levels in RASFs. Furthermore, altering FoxC1 expression did not significantly change GSK3β and pGSK3β levels. FoxC1 overexpression promoted proliferation, migration, invasion and proinflammatory cytokine (interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α)) production and reduced anti-inflammatory cytokine (IL-10) levels in RASFs. FoxC1 bound to the β-catenin promoter, and β-catenin mediated the FoxC1-induced pathological changes. We also observed downregulated microRNA (miR)-141-3p expression in SFs from both RA patients and CIA rats and further found that miR-141-3p bound to the FoxC1 3′UTR and suppressed FoxC1 expression. Intra-ankle miR-141-3p agomir or FoxC1-specific siRNA injection hindered CIA development in rats.
Conclusions: FoxC1 and miR-141-3p participate in RA pathogenesis by mediating inflammation and SF proliferation, migration, and invasion and thus could be novel targets for RA therapy as a nonimmunosuppressive approach.
Keywords: rheumatoid arthritis, FoxC1, miR-141-3p, synovial fibroblasts, collagen-induced arthritis
 Global reach, higher impact
Global reach, higher impact