13.3
Impact Factor
Theranostics 2020; 10(13):5749-5762. doi:10.7150/thno.41894 This issue Cite
Research Paper
Rescuing Dicer expression in inflamed colon tissues alleviates colitis and prevents colitis-associated tumorigenesis
1. Department of Gastroenterology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, Zhejiang, P.R. China
2. Digestive Cancer Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, Zhejiang, P.R. China
3. Key Laboratory of Diagnosis and Treatment of Severe Hepato-Pancreatic Diseases of Zhejiang Province, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, Zhejiang, P.R. China
4. Department of Dermato-Venereology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, Zhejiang, P.R. China
5. Department of Gynecology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, Zhejiang, P.R. China
6. Department of Gastroenterology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, P.R. China
7. Department of Gastroenterology, The Sixth People's Hospital of Chongqing, Chongqing 404100, P.R. China
8. Department of Hepatobiliary Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, P.R. China
9. Department of Bioengineering, College of Bioengineering, Chongqing University, Key Laboratory of Biorheological Science and Technology, Ministry of Education, Chongqing 400030, P.R. China
10. Department of Pathology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang, P.R. China
11. Department of Pathology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, Zhejiang, P.R. China
#These authors contributed equally.
Received 2019-11-7; Accepted 2020-4-4; Published 2020-4-27
Abstract
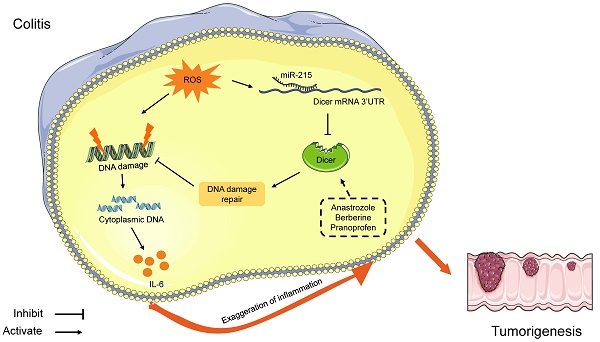
Chronic inflammation is known to promote carcinogenesis; Dicer heterozygous mice are more likely to develop colitis-associated tumors. This study investigates whether Dicer is downregulated in inflamed colon tissues before malignancy occurs and whether increasing Dicer expression in inflamed colon tissues can alleviate colitis and prevent colitis-associated tumorigenesis.
Methods: Gene expression in colon tissues was analyzed by immunohistochemistry, immunoblots, and real-time RT-PCR. Hydrogen peroxide or N-acetyl-L-cysteine was used to induce or alleviate oxidative stress, respectively. Mice were given azoxymethane followed by dextran sulfate sodium to induce colitis and colon tumors. Berberine, anastrozole, or pranoprofen was used to rescue Dicer expression in inflammatory colon tissues.
Results: Oxidative stress repressed Dicer expression in inflamed colon tissues by inducing miR-215 expression. Decreased Dicer expression increased DNA damage and cytosolic DNA and promoted interleukin-6 expression upon hydrogen peroxide treatment. Dicer overexpression in inflamed colon tissues alleviated inflammation and repressed colitis-associated carcinogenesis. Furthermore, we found that anastrozole, berberine, and pranoprofen could promote Dicer expression and protect cells from hydrogen peroxide-induced DNA damage, thereby reducing cytosolic DNA and partially repressing interleukin-6 expression upon hydrogen peroxide treatment. Rescuing Dicer expression using anastrozole, berberine, or pranoprofen in inflamed colon tissues alleviated colitis and prevented colitis-associated tumorigenesis.
Conclusions: Dicer was downregulated in inflamed colon tissues before malignancy occurred. Decreased Dicer expression further exaggerated inflammation, which may promote carcinogenesis. Anastrozole, berberine, and pranoprofen alleviated colitis and colitis-associated tumorigenesis by promoting Dicer expression. Our study provides insight into potential colitis treatment and colitis-associated colon cancer prevention strategies.
Keywords: Dicer, colitis-associated carcinogenesis, anastrozole, berberine, pranoprofen
Introduction
Dicer is a key component of the RNA interference pathway and is essential for the biogenesis of microRNAs (miRNAs) and small interfering RNAs (siRNAs) [1]. Recent evidence indicates that Dicer is frequently downregulated in tumor tissues, and that decreased Dicer expression promotes carcinogenesis [1, 2]. Somatic mutations in Dicer have been identified in various cancer types [1, 3, 4], and heterozygous germline Dicer mutations have been shown to increase the risk of a variety of tumors, particularly in the lungs, kidneys, ovaries, and thyroid [1, 5]. Although Dicer has been proposed to function as a haploinsufficient tumor suppressor [3], other studies have indicated that the full loss of Dicer does not preclude carcinogenesis [6, 7].
Global control of protein synthesis is crucial for cancer development and progression, as highly proliferating cancer cells require increased protein synthesis [8]. Decreased Dicer expression may promote protein synthesis through several mechanisms. First, Dicer is essential for miRNA biogenesis, and miRNAs repress protein synthesis by inhibiting translation or degrading their target mRNAs [1]; therefore, decreased Dicer expression may lead to increased protein synthesis due to globally impaired miRNA expression [2, 9, 10]. Second, Dicer can process full-length tRNA into small fragments called tRNA-derived small RNA (tsRNA) that inhibit protein translation [11, 12], therefore, decreased Dicer expression may lead to enhanced protein synthesis due to increased full-length tRNA levels and decreased tsRNA levels. Lastly, Dicer can process 7SL RNA into small fragments, which impair signal recognition particle (SRP) formation and thereby inhibit SRP-mediated protein targeting [13, 14]. SRP couples the synthesis of nascent proteins to their correct cellular destinations [15]; therefore, decreased Dicer expression may promote localization of nascent proteins to their correct cellular destinations. Altogether, these findings indicate that decreased Dicer expression may promote carcinogenesis by increasing protein synthesis.
Dicer is essential for DNA repair, and decreased Dicer expression has been shown to reduce the efficiency of DNA repair and leads to accumulation of DNA damage [16-21]. DNA damage can promote inflammation by inducing the expression of NKG2D ligands; upregulation of NKG2D ligands can initiate immune-mediated tissue damage and induce inflammation [22, 23]. Moreover, DNA damage leads to the accumulation of DNA and micronuclei in the cytoplasm; this cytosolic DNA triggers the production of proinflammatory cytokines such as type I interferons and interleukin-6 (IL-6) [24, 25]. Inflammation is indispensable in neoplastic processes and promotes cancer initiation and the subsequent proliferation, survival, and migration of cancer cells [26]. Knockout of Dicer in intestinal epithelial cells was found to induce inflammation, and Dicer heterozygous mice were more likely to develop colitis-associated tumors [27]. Therefore, in addition to its mutational consequences, decreased Dicer expression may promote tumorigenesis by inducing inflammation.
It has been reported that Dicer mRNA levels are slightly decreased in colon cancer tissues but significantly increased in rectum cancer tissues compared with normal mucosa tissues [28]. Moreover, Dicer mRNA levels in stage I and stage II colorectal tumor tissues are lower than in stage III and stage IV tumor tissues [29]. Inflammation plays pivotal roles in the development of colitis-associated colon cancer [26, 30]. However, the dynamic pattern of Dicer expression during colitis-associated carcinogenesis remains unclear. Accordingly, in the current study, we examined whether Dicer is downregulated in inflamed colon tissues before a malignant change occurs. In addition, we investigated whether rescue of Dicer expression in inflamed colon tissues alleviates colitis and colitis-associated tumorigenesis.
Methods
Cell culture and drug treatment
FHC, CCD-18Co, and THP-1 cell lines were purchased from the American Type Culture Collection (Manassas, VA) and HEK293T was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). CCD-18Co and HEK293T were grown in DMEM (Hyclone, Logan, UT) supplemented with 10% FBS, while FHC was grown in RPMI 1640 (Hyclone) supplemented with 10% FBS. THP-1 cells were cultured in RPMI 1640 containing 10% FBS and 50 μM β-mercaptoethanol. Cells were cultured at 37 °C in a 5% CO2 humidified incubator. All cell lines were mycoplasma free, and cells passaged in our laboratory > 6 months after receipt were authenticated by genetic profiling using polymorphic short tandem repeat loci. To identify drugs that can promote Dicer expression, we screened a drug library (Targetmol, Wellesley Hills, MA), which contains 1800 US-FDA approved drugs. Briefly, cells were seeded in 6-well plates to reach 80% confluence, and then treated with different drugs at a concentration of 30 μM. Dicer protein levels were determined using western blotting 24 h after treatment.
Human colon tissue specimens
In total, 91 normal colon tissue samples and 102 inflammatory bowel diseases (IBD) colon tissue samples were collected from patients who underwent enteroscopy and biopsy at the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China). Informed consent was obtained from all patients for the collection and use of clinical samples, and the study was approved by the Scientific Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Samples were either rapidly frozen using liquid nitrogen or fixed in formalin solution. Rapidly frozen samples were stored at -80 °C to further extract RNA and protein for miR-215 and Dicer quantification.
Mouse studies
Acute colitis was induced in six-week-old male mice by oral administration of 3% dextran sulfate sodium (DSS; MP Biomedicals, Santa Ana, CA) in drinking water for 7 days, followed by 2 days of normal drinking water. All mice were euthanized on day 9.
To induce colitis-associated colon cancers, six-week-old male C57BL/6 mice were injected intraperitoneally with 12.5 mg/kg azoxymethane (AOM; Sigma-Aldrich, St. Louis, MO). After 5 days, chronic colitis was induced by 3 cycles of DSS. One cycle of DSS was defined as 5 days of DSS administration followed by a recovery period of 16 days on normal drinking water. All mice were euthanized on day 92. Control mice received normal drinking water throughout the duration of the experiment.
To introduce adenoviruses or lentiviruses to mouse colon tissues, mice were anesthetized and given an intrarectal enema of 100 μL 50% ethanol. Three hours after the enema, 100 μL lentivirus solution containing 108 titers lentivirus or 109 adenovirus was intrarectally instilled from the mouse anus. The mice were inverted for 30 s after intrarectal administration ofvirus to prevent leakage. The mouse Dicer overexpression adenovirus or control adenovirus was customized from Cyagen Biosciences Inc. (Guangzhou, China). Mouse Dicer shRNA or control lentivirus was purchased from Origene (Rockville, MD).
To relieve oxidative stress in inflamed tissues, NAC (Sigma-Aldrich) was added to drinking water at a final concentration of 2%.
All mice were reared and handled in accordance with the Institutional Guidelines on Animal Usage and Maintenance of Wenzhou Medical University.
Plasmids, siRNAs, miRNAs, and transfection
Cells were transfected with siRNAs, miRNAs, or plasmids using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. miRNA mimics, the miR-215 inhibitor (anti-miR-215), and the respective negative controls (miR-Con and anti-miR-Con) were purchased from RiboBio (Guangzhou, China). Control and Dicer siRNAs were obtained from Life Technologies and the sequences were described previously [16]. The human Dicer overexpression plasmid pDESTmycDICER (pDicer) was obtained from Addgene (Cambridge, MA).
Western blotting
The total cell lysate was subjected to SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Millipore). Blots were incubated with primary antibodies, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies and detection with ECL plus reagents (GE Healthcare, Chicago, IL). Primary antibodies used were anti-Dicer (ab14601; Abcam, Cambridge, UK), anti-γ-Tubulin (BM1606; BosterBio, Wuhan, China), and anti-GAPDH (2188; Cell Signaling Technology, Danvers, MA). Relative Dicer expression levels were calculated via densitometry and normalized to GAPDH expression using Image J software (http://rsb.info.nih.gov/ij/).
Cell proliferation assay
Cell proliferation was assessed using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS) kit (Promega, Madison, WI) according to the manufacturer's instructions.
Immunohistochemistry, hematoxylin and eosin (HE) staining, and TUNEL assay
Immunohistochemistry was performed as described previously [18]. To assay inflammatory cell infiltration, the hydrated tissue sections were stained with HE, dehydrated in ethanol, cleared in xylene, and mounted on coverslips. Inflammatory cell infiltration was scored as grade 0, 0%; grade 1, 1%-20%; grade 2, 21%-40%; grade 3, 41%-60%; grade 4, 61%-80%; and grade 5, 81%-100%. To assay neutrophil infiltration, the tissue sections were stained with anti-Ly-6G antibody (ab25377; Abcam).
Apoptosis in human and mouse colon tissues was detected using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit (Beyotime, Shanghai, China) as described previously [31].
Annexin V-FITC/PI apoptosis assay
Cell apoptosis was detected using the Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime) according to manufacturer's instructions.
Immunofluorescence
Immunofluorescence was performed as described previously [16]. Primary antibodies used in this study included anti-γ-H2AX antibody (2577; Cell Signaling Technology) and anti-double stranded DNA monoclonal antibody (MAB1293; Millipore).
Real-time RT-PCR
Real-time RT-PCR was performed as described previously [16]. The primer sequences were as follows, Dicer (human), 5'-TCCACGAGTCACAATCAACACGG-3' and 5'-GGGTTCTGCATTTAGGAGCTAGATGAG-3'; IL-6 (human), 5'-CAATCTGGATTCAATGAGGAGAC-3' and 5'-CTCTGGCTTGTTCCTCACTACTC-3'; GAPDH (human), 5'-ATGACATCAAGAAGGTGGTG-3' and 5'-CATACCAGGAAATGAGCTTG-3'; Dicer (mouse), 5'-GCCAAGAAAATACCAGGTTGAGC-3' and 5'-GCGATGAACGTCTTCCCTGAG-3'; GAPDH (mouse), 5'-ACGGCCGCATCTTCTTGTGCA-3' and 5'-ACGGCCAAATCCGTTCACACC-3'. To evaluate miR-215 expression, real-time RT-PCR was performed using the bulge-loop miRNA qPCR primer set (RiboBio, Guangzhou, China) according to manufacturer's instructions.
Dual-luciferase assays
Dual-luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega) as described previously [31].
Comet assay
Comet assay was performed as described previously [16, 31].
Detection of 8-hydroxydesoxyguanosine
The colon tissues were homogenized with PBS and centrifuged (12000 × g for 15 min at 4 °C). The supernatants were collected to determine the total protein concentration using a BCA protein assay kit (Beyotime). The levels of 8-Hydroxydesoxyguanosine (8-OHdG) in the supernatants were measured using the Enzyme-Linked Immunosorbent Assay Kit For 8-OHdG (Cloud-Clone Corp., Houston, TX) according to the manufacturer's instructions. The results are expressed as μg of 8-OHdG per mg of total protein (μg/mg protein).
Statistical analysis
All experimental data are presented as means ± SEM of at least three independent experiments. The number of mice per group is indicated in the figures, and significant differences between groups were determined using Student's t-test when variances were equal. When variances were unequal, Welch's t-test was used. The correlation between two variables was assessed by Spearman correlation analysis. T-tests were performed using GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA) and Spearman correlation analysis was performed using SPSS 22.0 software (IBM, Armonk, NY). P-values < 0.05 were considered statistically significant.
Results
Dicer is downregulated in inflamed colon tissues before malignancy occurs
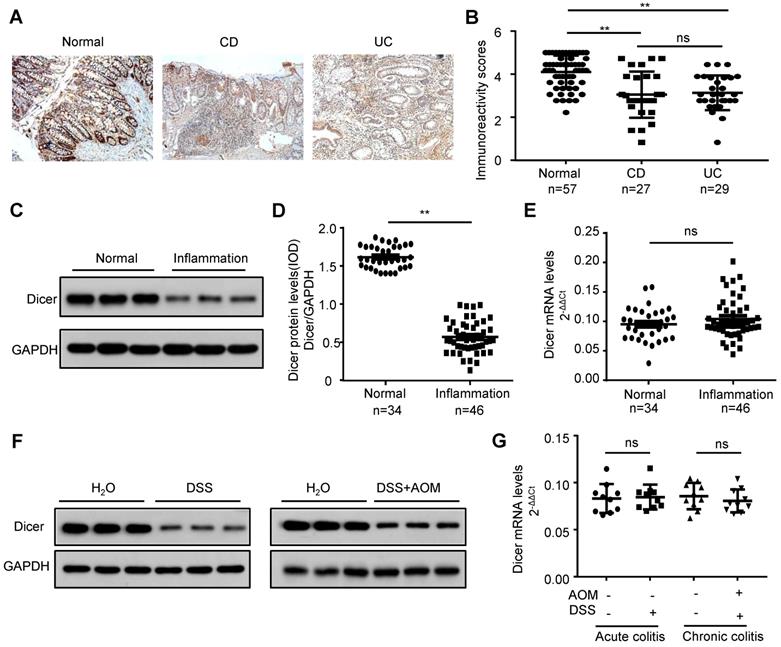
To investigate whether Dicer is downregulated in inflamed colon tissues before a malignant change occurs, we first examined Dicer expression in paraffin-embedded colon tissues from 56 patients with IBD (27 Crohn's disease and 29 ulcerative colitis) and 57 controls. Immunochemistry revealed that Dicer was downregulated in inflamed colon tissues compared with control colon tissues (Figure 1A-B). Using frozen inflamed colon tissues from another 46 patients with IBD and 34 controls, we found that Dicer was downregulated at the protein level, but not at the mRNA level (Figure 1C-E). Moreover, we found that Dicer was also downregulated at the protein level but not at the mRNA level in inflamed colon tissues derived from DSS-induced acute or AOM plus DSS-induced chronic colitis mouse models (Figure 1F-G). Collectively, these findings suggest that Dicer expression is downregulated in inflamed colon tissues before malignancy occurs.
Oxidative stress represses Dicer expression in inflamed colon tissues
Inflammatory conditions inevitably lead to oxidative stress [32]. To investigate whether inflammation represses Dicer expression via oxidative stress, we treated the human colon epithelial cell line FHC, the human colon myofibroblast cell line CCD-18Co, and the human macrophage cell line THP-1 with hydrogen peroxide (H2O2). Our results revealed that H2O2 inhibited Dicer protein, but not mRNA, expression in a dose- and time-dependent manner (Figure 2A and Figure S1A-D). Treatment with N-acetyl-L-cysteine (NAC), an effective antioxidant, partially rescued the H2O2-induced Dicer downregulation (Figure 2B and Figure S1E). Moreover, administration of NAC to colitis mouse models partially rescued Dicer expression in inflamed colon tissues (Figure 2C-D). As expected, 8-hydroxy-2'-deoxyguanosine (8-OHdG), a biomarker of oxidative stress and oxidative DNA damage [33], was increased in human and mouse inflamed colon tissues compared with control colon tissues (Figure S2). Moreover, we detected an inverse correlation between Dicer protein levels and 8-OHdG levels in human inflamed colon tissues (Figure 2E). Therefore, these results indicate that oxidative stress represses Dicer expression in inflamed colon tissues.
Decreased Dicer expression in inflamed colon tissues. (A, B) Immunohistochemistry of Dicer expression in 56 inflamed colon tissues and 57 normal colon tissues. Representative immunohistochemistry images (A) and semi-quantitative evaluation (B) of Dicer protein expression. (C-E) Analysis of Dicer expression in 46 inflamed colon tissues and 34 normal colon tissues. Representative western blotting images of Dicer protein levels in three normal colon tissues and three inflamed colon tissues (C). Dicer and GAPDH protein levels were determined via densitometry using ImageJ and are represented as IOD (D). Dicer mRNA levels were determined by real-time RT-PCR (E). (F, G) Dicer expression in colon tissues derived from control mice, DSS-induced acute, or AOM/DSS-induced chronic colitis mice was determined by western blotting (F) and real-time RT-PCR (n = 10 mice per group) (G). Data represent the means ± SEM. **P < 0.01. ns, not significant. AOM: azoxymethane; CD: Crohn's disease; DSS: dextran sulfate sodium; IOD: integrated optical density; UC: ulcerative colitis.
Oxidative stress represses Dicer expression in inflamed colon tissues by inducing miR-215 expression
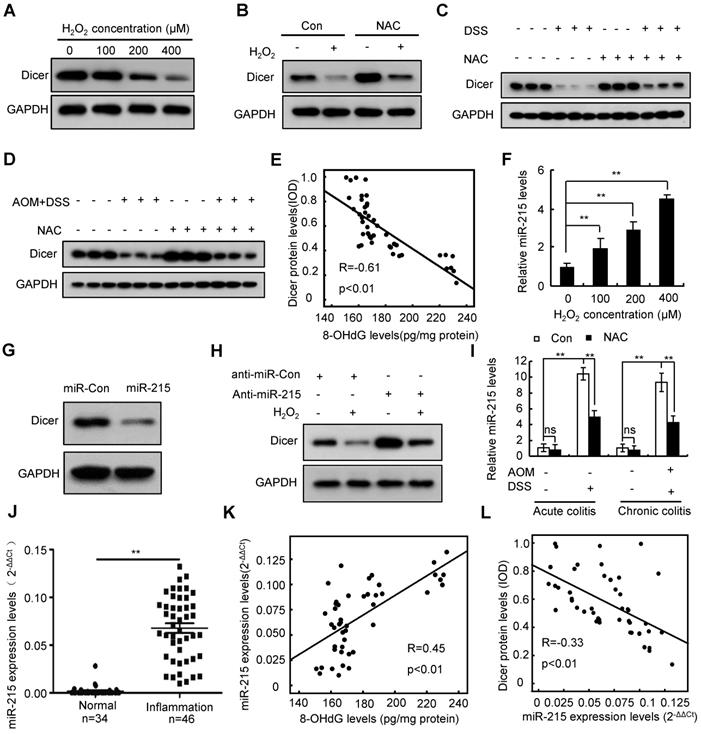
Analysis of previously published microarray data [34] revealed that H2O2 induced miR-215 expression in a time-dependent manner in mouse fibroblasts (Figure S3A). Quantitative RT-PCR confirmed that H2O2 treatment dose-dependently induced miR-215 expression in FHC, CCD-18Co, and THP-1 cells (Figure 2F and Figure S3B). Targetscan prediction revealed that there are three potential miR-215 binding sites on the 3′-untranslated region (UTR) of Dicer (Figure S3C; top panel). To examine whether miR-215 regulates Dicer expression, we performed a dual luciferase assay using three reporter vectors consisting of the luciferase coding sequence followed by different fragments of the 3′-UTR of Dicer (Figure S3C; bottom panel). As shown in Figure S3D, miR-215 repressed luciferase activity of all three reporters. Transient transfection of miR-215 decreased Dicer protein expression but did not affect Dicer mRNA expression (Figure 2G and Figure S3E-F). miR-215 inhibitors partially rescued Dicer protein but not mRNA expression upon H2O2 treatment (Figure 2H and Figure S3G-H). Collectively, these findings indicate that H2O2 treatment represses Dicer expression by inducing miR-215 expression.
We then investigated whether oxidative stress in inflamed colon tissues represses Dicer expression by inducing miR-215 expression. As shown in Figure 2I, miR-215 was upregulated in inflamed colon tissues of colitis mouse models; conversely, administration of NAC partially repressed this miR-215 upregulation. Moreover, miR-215 expression was significantly upregulated and positively correlated with 8-OHdG levels in human inflamed colon tissues (Figure 2J-K). Expression levels of Dicer protein were inversely correlated with miR-215 levels in human inflamed colon tissues (Figure 2L). Together, these results indicate that oxidative stress represses Dicer expression by inducing miR-215 expression.
Oxidative stress represses Dicer expression in inflamed colon tissues by inducing miR-215 expression. (A) FHC cells were treated with different doses of H2O2, Dicer protein level was determined 24 h after treatment. (B) FHC cells were treated with 400 µM H2O2 and 2 mM NAC; Dicer protein levels were determined 24 h after treatment. (C, D) Dicer expression in colon tissues derived from DSS-induced acute (C) or AOM/DSS-induced chronic (D) colitis mouse models with or without NAC treatment was determined by western blotting. (E) Correlation between 8-OHdG levels and Dicer levels in 46 inflamed colon tissues. (F) FHC cells were treated with different doses of H2O2 for 24 h, and the level of miR-215 was quantified. (G) FHC cells were transfected with miR-215 mimics, and Dicer protein levels were determined 48 h post-transfection. (H) FHC cells were transfected with miR-215 inhibitors, and 400 µM H2O2 was added to the culture medium 24 h after transfection. Dicer protein levels were determined 24 h after H2O2 treatment. (I) miR-215 levels were quantified in colon tissues derived from acute or chronic colitis mouse models treated with or without NAC; n = 8 mice per group. (J) miR-215 levels were quantified in 34 normal control colon tissues and 46 inflamed colon tissues. (K) Correlation between 8-OHdG levels and miR-215 levels in 46 inflamed colon tissues. (L) Correlation between miR-215 levels and Dicer protein levels in 46 inflamed colon tissues. Data represent the means ± SEM. **P < 0.01. ns, not significant. AOM: azoxymethane; DSS: dextran sulfate sodium; NAC: N-acetyl-L-cysteine
Decreased Dicer expression sensitizes cells to oxidative stress-induced DNA damage and apoptosis
As oxidative stress induces DNA damage [32], and Dicer plays important roles in DNA repair [16-21], we examined whether decreased Dicer expression sensitizes cells to oxidative stress-induced DNA damage. Comet assays and immunostaining with γ-H2AX antibody indicated that H2O2 induced more DNA damage in Dicer knockdown cells than in control cells (Figure S4). Cell proliferation and apoptosis assays also revealed that Dicer knockdown sensitized cells to H2O2 treatment (Figure S5A-B). TUNEL staining revealed that apoptosis was increased in inflamed colon tissues (Figure S5C) and that Dicer knockdown further increased apoptosis in DSS-induced inflamed colon tissues (Figure S5D). Interestingly, we found an inverse correlation between expression levels of Dicer protein and apoptosis levels in human inflamed colon tissues (Figure S5E).
Decreased Dicer expression increases cytosolic DNA and promotes IL-6 expression upon H2O2 treatment
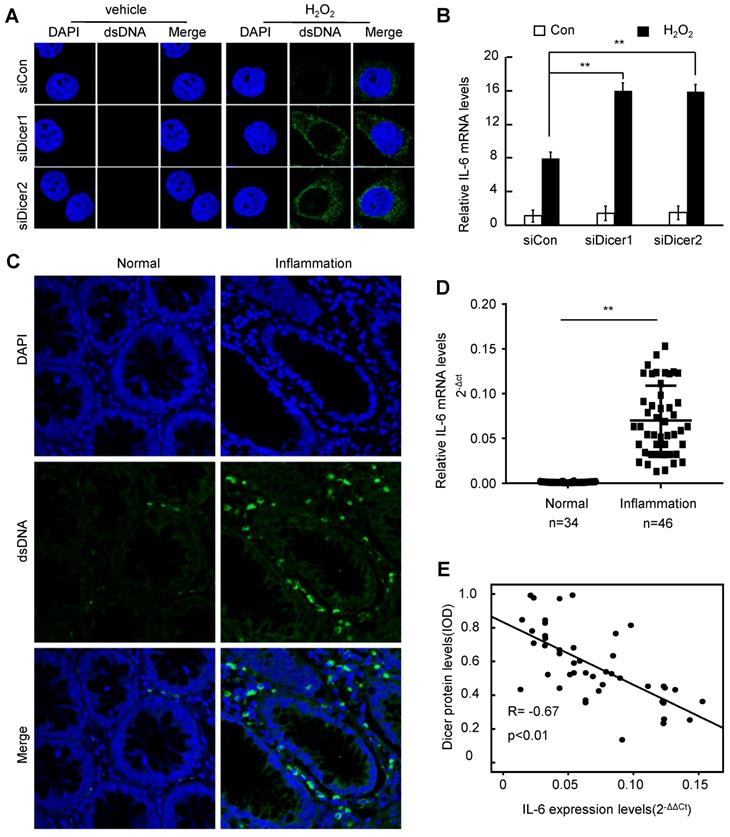
DNA damage leads to accumulation of cytosolic DNA, triggering the production of IL-6, which promotes inflammatory disease and cancer [24, 25, 35]. Treatment with H2O2 increased cytosolic DNA in different cells, and Dicer knockdown further increased the H2O2-induced cytosolic DNA accumulation (Figure 3A and Figure S6A). Consistently, we found that cytosolic DNA was accumulated in inflamed human colon tissues (Figure 3C). Compared with control cells, Dicer-knockdown cells expressed higher levels of IL-6 upon H2O2 treatment (Figure 3B and Figure S6B). Furthermore, we found that IL-6 expression was increased in human inflamed colon tissues compared with normal healthy colon tissues (Figure 3D), and an inverse correlation between the expression levels of Dicer protein and the mRNA levels of IL-6 was observed in human inflamed colon tissues (Figure 3E). Thus, these results indicate that decreased Dicer expression promotes the accumulation of cytosolic DNA upon H2O2 treatment, thereby enhancing H2O2-induced expression of IL-6.
Decreased Dicer expression potentiates DSS-induced inflammation in colon tissues and promotes colitis-associated carcinogenesis
Consistent with the finding that heterozygous knockout of Dicer in intestinal epithelial cells increases inflammatory cell infiltration in colon tissues [27], we found that Dicer knockdown in the colon tissues of DSS-induced acute colitis mouse model promoted inflammation in terms of body weight loss, final colon length, inflammatory cell infiltration and cell apoptosis in colon tissues, and serum IL-6 levels (Figure S7, and Figure S5D). Moreover, Dicer knockdown in the colon tissues of AOM/DSS-induced colitis-associated colon cancer mouse model not only increased the severity of inflammation, but also promoted carcinogenesis (Figure S8).
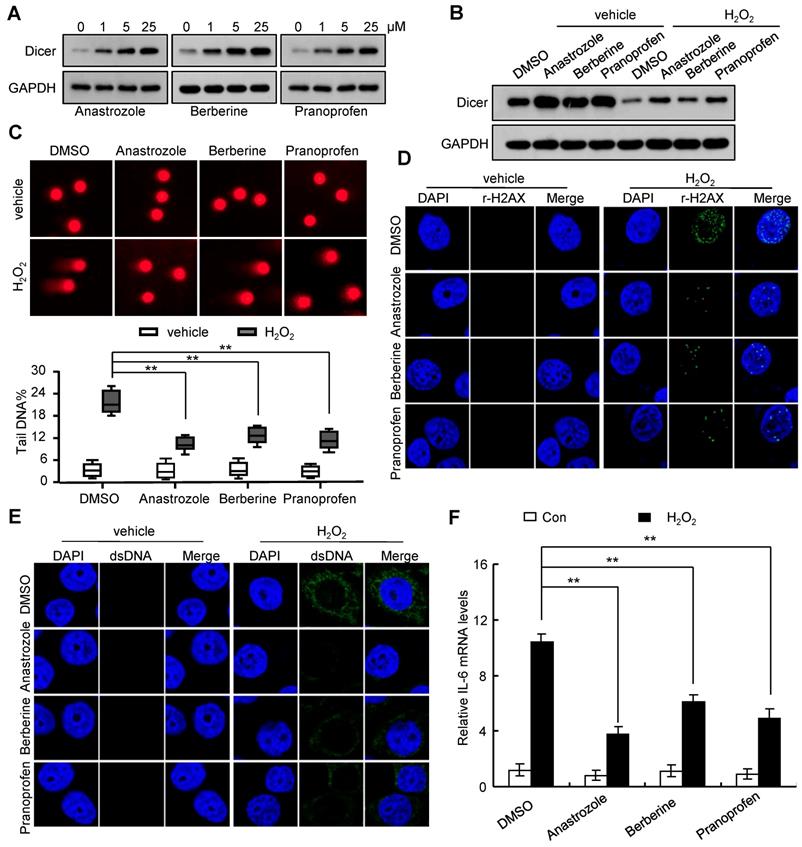
Anastrozole, berberine, and pranoprofen enhance Dicer expression and decrease H2O2-induced IL-6 expression
Consistent with our previous report that Dicer overexpression promoted DNA repair [18], we found that Dicer overexpression reduced H2O2-induced DNA damage, decreased the H2O2-induced increase in cytosolic DNA, and alleviated the H2O2-induced IL-6 expression (Figure S9, and Figure S10). By screening a drug library, we found that anastrozole, berberine, and pranoprofen could enhance Dicer expression (Figure 4A and Figure S11A). An increase in Dicer expression induced by anastrozole, berberine, or pranoprofen reduced sensitivity to H2O2-induced DNA damage (Figure 4B-D and Figure S11B-D). Consequently, treatment with anastrozole, berberine, or pranoprofen decreased the H2O2-induced increase in cytosolic DNA and IL-6 expression (Figure 4E-F and Figure S12). Silencing Dicer expression partially abrogated these effects of anastrozole, berberine, and pranoprofen (Figure S13, and Figure S14). Collectively, these findings reveal that anastrozole, berberine, or pranoprofen can prevent H2O2-induced DNA damage by promoting Dicer expression.
Rescue of Dicer expression in inflamed colon tissues alleviates colitis and represses colitis-associated tumorigenesis
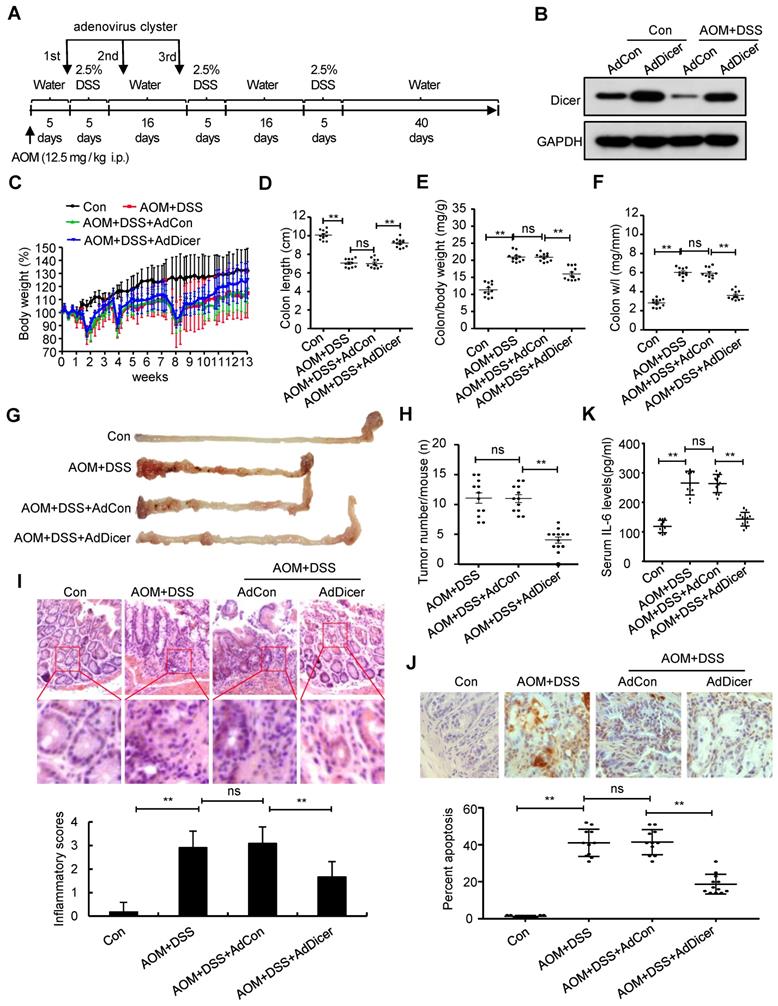
We next investigated whether rescue of Dicer expression in inflamed colon tissues alleviates colitis and represses colitis-associated tumorigenesis. Adenovirus-mediated Dicer overexpression in the colon tissues of acute colitis mouse models alleviated inflammation in terms of body weight loss, final colon length, inflammatory cell infiltration, and cell apoptosis, as evidenced by histological analysis and serum IL-6 levels (Figure S15). Dicer overexpression in the colon tissues of chronic colitis mouse models not only reduced the severity of inflammation, but also markedly reduced the number of colon tumors (Figure 5).
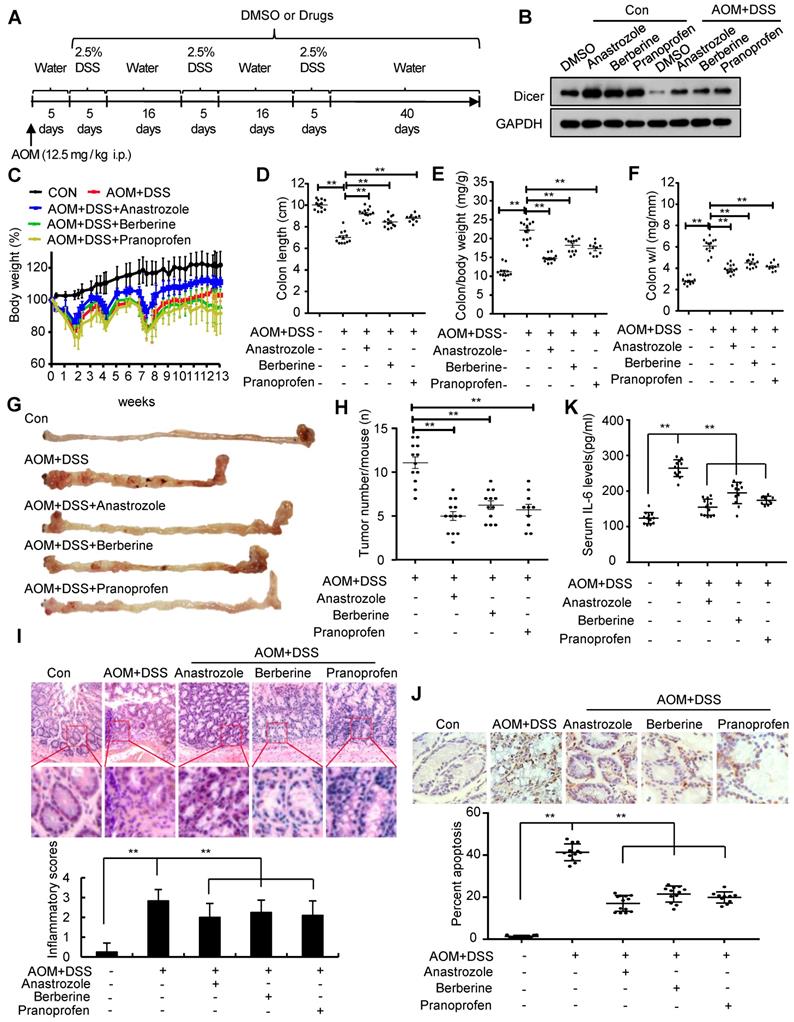
Administration of anastrozole, berberine, or pranoprofen to colitis mouse models rescued Dicer expression in inflamed colon tissues (Figure 6A-B and Figure S16A-B). Pharmacological rescue of Dicer expression in an acute colitis mouse model alleviated inflammation in terms of final colon length, inflammatory cell infiltration, and cell apoptosis in colon tissues, and serum IL-6 levels (Figure S16C-J, and Figure S17). Similar to Dicer overexpression, pharmacological rescue of Dicer expression in a chronic colitis mouse model reduced inflammation severity and colon tumor formation (Figure 6C-J and Figure S18). Silencing Dicer expression in colon tissues partially abrogated the effects of anastrozole, berberine, and pranoprofen on inflammation and inflammation-associated colon cancers (Figure S19, and Figure S20), thereby indicating that these drugs alleviate colitis and prevent colitis-associated colon cancers via upregulating Dicer expression.
Decreased Dicer expression leads to increased cytosolic DNA and IL-6 expression after H2O2 treatment. (A, B) FHC cells were transfected with control or Dicer siRNAs, and 400 µM H2O2 was added to the culture medium 24 h post-transfection; cytosolic dsDNA levels (A) as well as IL-6 mRNA levels (B) were determined 24 h after H2O2 treatment. (C) Representative confocal microscopy image of immunocytochemistry for cytosolic DNA in inflamed and control colon tissues. (D) IL-6 mRNA levels were quantified in 34 normal colon tissues and 46 inflamed colon tissues. (E) Correlation between Dicer protein levels and IL-6 mRNA levels in 46 inflamed colon tissues. Data represent the means ± SEM. **P < 0.01. ns, not significant.
Anastrozole, berberine, or pranoprofen enhance Dicer expression and decrease H2O2-induced IL-6 expression. (A) FHC cells were treated with different doses of anastrozole, berberine, or pranoprofen; Dicer protein levels were determined 24 h after treatment. (B-F) FHC cells were treated with 800 µM H2O2, 800 µM H2O2 + 5 µM anastrozole, 800 µM H2O2 + 5 µM berberine, or 800 µM H2O2 + 5 µM pranoprofen for 24 h. Dicer protein levels were determined by western blotting (B), DNA damage was assayed by comet assays (C) or immunostaining with γ-H2AX (D), cytosolic dsDNA levels were determined by immunostaining with anti-dsDNA antibody (E), and IL-6 mRNA levels were quantified by real-time RT-PCR (F). Data represent the means ± SEM. **P < 0.01. ns, not significant.
Discussion
In this study, we found that oxidative stress in inflamed colon tissues repressed Dicer expression by inducing miR-215 expression, and that decreased Dicer expression sensitized cells to oxidative stress-induced DNA damage and promoted IL-6 expression in inflamed colon tissues. These findings indicate that inflammation represses Dicer expression, and decreased Dicer expression further exaggerates inflammation. Moreover, we demonstrated that adenovirus-mediated Dicer overexpression in inflamed colon tissues alleviates inflammation and represses colitis-associated tumorigenesis. Collectively, our findings suggest that Dicer downregulation in inflamed tissues drives a local auto-amplification loop that leads to uncontrolled inflammation, which may promote cancer initiation and progression [26].
Dicer overexpression reduces AOM/DSS-induced inflammation in colon tissues and alleviates colitis-associated carcinogenesis. (A) Schematic of experimental setup: six-week-old male C57BL/6 mice were injected intraperitoneally with 12.5 mg/kg AOM, followed by three cycles of 2.5% DSS treatment. To increase Dicer expression in colon tissues, adenovirus containing the Dicer expression cassette was intrarectally administrated to mice three times. All mice were euthanized 92 days after AOM injection. Mice that received normal drinking water and were not instilled with adenovirus were used as control. (B) Dicer expression in colon tissues was determined by western blotting. (C) Relative body weight curves, (D) colon length, (E) colon/body weight ratio, (F) colon weight/length (w/l) ratio, (G) representative images of mouse gross colon, and (H) tumor numbers in the mouse colorectum. (I) Representative HE-stained colon sections showing inflammatory infiltrate (upper panel) and inflammatory scores (lower panel). (J) Representative images of TUNEL-stained tissue sections (upper panel) and the percentage of apoptotic cells in colon tissues (lower panel). (K) Serum IL-6 levels. Data represent the means ± SEM of at least 11 mice per group. **P < 0.01. ns, not significant. AdCon: control adenovirus; AdDicer: Dicer overexpression adenovirus; AOM: azoxymethane; DSS: dextran sulfate sodium; HE: hematoxylin and eosin; TUNEL: transferase dUTP nick end labeling
Upregulation of Dicer expression by anastrozole, berberine, or pranoprofen alleviates inflammation and prevents colitis-associated carcinogenesis. (A) Schematic of experimental setup: six-week-old male C57BL/6 mice were injected intraperitoneally with 12.5 mg/kg AOM, followed by three cycles of 2.5% DSS treatment. To rescue Dicer expression in inflamed colon tissues, 20 mg/kg anastrozole, 28 mg/kg berberine, or 16 mg/kg pranoprofen was added to the drinking water. (B) Dicer expression in colon tissues was determined by western blotting. (C) Relative body weight curves, (D) colon length, (E) colon/body weight ratio, (F) colon weight/length (w/l) ratio, (G) representative images of mouse gross colon, and (H) tumor numbers in the mouse colorectum. (I) Representative HE-stained colon sections showing inflammatory infiltrate (upper panel) and inflammatory scores (lower panel). (J) Representative images of TUNEL-stained tissue sections (upper panel) and the percentage of apoptotic cells in colon tissues (lower panel). (K) Serum IL-6 levels. Data represent the means ± SEM of at least 8 mice per group. **P < 0.01. HE: hematoxylin and eosin; TUNEL: transferase dUTP nick end labeling
miR-215 is a p53 target that is upregulated in response to DNA damage [36]. It functions as a tumor suppressor through various mechanisms, e.g., inducing cell cycle arrest and promoting cancer stem cell differentiation [36-38]. Low miR-215 expression in renal cell carcinoma tissues is associated with a significantly reduced disease-free survival time [38]. However, other studies have demonstrated that miR-215 has oncogenic functions as it targets tumor suppressors [39, 40] and that a high expression of miR-215 in colon cancer tissues is associated with poor overall survival [41]. Our findings revealed that miR-215 might function as an oncogene in inflammation-driven carcinogenesis by repressing Dicer expression. The effect of oxidative stress on miR-215 expression was multifaceted. As DNA damage induces miR-215 transcription [36], oxidative stress can induce DNA damage and therefore promote miR-215 transcription. Consistent with this, we found that H2O2-treatment not only increased the levels of mature miR-215, but also increased the levels of pri-miR-215 and pre-miR-215 (Figure S21), suggesting that oxidative stress may induce miR-215 transcription. However, given that miR-215 can repress Dicer expression, and Dicer is essential for the procession of pre-miR-215 into mature miR-215, Dicer downregulation may facilitate a feedback to prevent excessive miR-215 upregulation upon oxidative stress. Indeed, we found that the increase of mature miR-215 was less than that of pri-miR-215 and pre-miR-215 upon H2O2-treatment (Figure S21). Moreover, we found that berberine, anastrozole, and pranoprofen did not affect expression and function of miR-215 (Figure S22), indicating that these drugs promote Dicer expression via a miR-215-independent mechanism.
Anastrozole is a nonsteroidal aromatase inhibitor that can reversibly bind to the aromatase enzyme and block the conversion of androgens to estrogens, and is used to treat or prevent breast cancer [42]. In the present study, we found that anastrozole could be used to alleviate inflammation in colon tissues and prevent colitis-associated colon cancer by enhancing Dicer expression. Although the molecular mechanisms underlying how anastrozole induces Dicer expression remains to be elucidated, the mechanisms are not likely to be dependent on the activity of aromatase because letrozole, another nonsteroidal aromatase inhibitor, does not affect Dicer expression (Figure S21).
Berberine, a natural plant product, has been shown to lower blood glucose and lipid levels and increase insulin sensitivity in numerous clinical trials [43, 44]. Preclinical data showed that berberine exerts anti-inflammatory and anti-cancer activities via different mechanisms [43, 44]. In this study, we uncovered a new molecular mechanism underlying the anti-inflammatory and anti-cancer activities of berberine, which alleviates oxidative stress-induced DNA damage by promoting Dicer expression, thereby relieving inflammation, and repressing inflammation-driven carcinogenesis. Our finding is consistent with that of a previous study wherein berberine repressed single-strand DNA cleavage induced by H2O2 and cytochrome c [45]. However, this is in contrast with another report where berberine was found to repress homologous recombination repair and induce DNA damage [46].
Pranoprofen, a nonsteroidal anti-inflammatory drug used in ophthalmology, inhibits cyclooxygenase and reduces the formation of prostaglandins [47]. We found that pranoprofen alleviates DNA damage upon oxidative stress by promoting Dicer expression, thereby relieving inflammation, and preventing colitis-associated carcinogenesis. Although we have not yet elucidated the molecular mechanisms underlying pranoprofen induction of Dicer expression, the mechanisms may not be dependent on the activity of cyclooxygenase because other cyclooxygenase inhibitors, including amfenac sodium monohydrate, naproxen, and rofecoxib, do not affect Dicer expression (Figure S23). Therefore, our findings uncovered a cyclooxygenase-independent anti-inflammatory activity of pranoprofen.
Although all the drugs tested, including anastrozole, berberine and pranoprofen, have been shown to alleviate inflammation in terms of final colon length, inflammatory cell infiltration and cell apoptosis in colon tissues, and serum IL-6 levels, their effects on mouse body weight were different, i.e., anastrozole prevented colitis-induced body weight loss, while berberine and pranoprofen had minimal effects on colitis-induced body weight loss (Figure 6C and Figure S16C, S17A, S18A). In a previous study, berberine was also shown to boost metabolism, improve glucose tolerance, and reduce body weight without altering food intake in db/db mice [48]. Given that adenovirus-mediated Dicer overexpression in inflamed colon tissues rescued colitis-induced body weight loss (Figure 5C, and Figure S15C), berberine may both positively and negatively regulate body weight of colitis mice as it can rescue colitis-induced body weight loss by increasing Dicer expression and can decrease body weight by promoting metabolism. Pranoprofen can also positively and negatively regulate body weight of colitis mice as it can prevent colitis-induced body weight loss by increasing Dicer expression and can decrease body weight by reducing food and water intake (Figure S24). Pranoprofen frequently causes gastrointestinal side effects [49], however, and may not be a good candidate for treating colitis. Therefore, further research or clinical trials should be conducted to test the efficacy of anastrozole and berberine in colitis treatment and colitis-associated colon cancer prevention.
Conclusion
In summary, we found that Dicer was downregulated in inflamed colon tissues before malignancy occurred. Decreased Dicer expression increased cytosolic DNA and promoted IL-6 expression upon oxidative stress. These findings suggest that Dicer downregulation in inflamed tissues drives a local auto-amplification loop that leads to uncontrolled inflammation, which may promote cancer initiation and progression. Moreover, we found that rescue of Dicer expression in inflamed colon tissues by berberine, anastrozole, or pranoprofen alleviated colitis and prevented colitis-associated tumorigenesis. Our study may shed light on potential colitis treatment and colitis-associated colon cancer prevention strategies.
Abbreviations
8-OHdG: 8-hydroxy-2'-deoxyguanosine; AOM: azoxymethane; DAB: 3,3′-diamino-benzidine; DSS: dextran sulfate sodium; HE: hematoxylin and eosin; HRP: horseradish peroxidase; H2O2: hydrogen peroxide; IBD: inflammatory bowel diseases; IL-6: interleukin-6; IOD: integrated optical density; NAC: N-acetyl-L-cysteine; PBS: phosphate-buffered saline; SRP: signal recognition particle; tsRNA: tRNA-derived small RNA; TUNEL: transferase dUTP nick end labeling; UTR: untranslated region.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 81773011, 81972648, 81572780 and 11832008) and the Zhejiang Provincial Natural Sciences Foundation (grant nos. LZ16H160004 and LY18H030008).The authors wish to thank Dr. Shanshan Lu and Dr. Kaiyan Yang (The First Affiliated Hospital of Wenzhou Medical University) for their assistance with pathology techniques, and Dr. Bin Tan (The Affiliated Children Hospital of Chongqing Medical University) for providing research facilities and technical assistance.
Author Contributions
K.F.T. conceived and devised the study. K.F.T., X.W., G.S., and X.C. designed the experiments and analysis. X.W., H.L., X.C., Z.W.H., Z.W., L.J.W., W.Y.W., S.Z., Q.H., Z.Z., Y.L., Y.J., and L.Z. performed the experiments and analyzed the data. G.S., R.O., J.G., W.Y., and Y.X. contributed reagents and materials. K.F.T. supervised the research and wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer. 2014;14:662-72
2. Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673-7
3. Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J. et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 2009;23:2700-4
4. Heravi-Moussavi A, Anglesio MS, Cheng SW, Senz J, Yang W, Prentice L. et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med. 2012;366:234-42
5. Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D. et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965
6. Ravi A, Gurtan AM, Kumar MS, Bhutkar A, Chin C, Lu V. et al. Proliferation and tumorigenesis of a murine sarcoma cell line in the absence of DICER1. Cancer Cell. 2012;21:848-55
7. Sekine S, Ogawa R, Ito R, Hiraoka N, McManus MT, Kanai Y. et al. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009;136:2304-15.e1 -4
8. Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10:254-66
9. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D. et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-8
10. Yoshikawa T, Wu J, Otsuka M, Kishikawa T, Suzuki N, Takata A. et al. Repression of microRNA function mediates inflammation-associated colon tumorigenesis. Gastroenterology. 2017;152:631-43
11. Haussecker D, Huang Y, Lau A, Parameswaran P, Fire AZ, Kay MA. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA. 2010;16:673-95
12. Sobala A, Hutvagner G. Small RNAs derived from the 5' end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013;10:553-63
13. Ren YF, Li G, Xue YF, Zhang XJ, Song YJ, Lv L. et al. Decreased dicer expression enhances SRP-mediated protein targeting. PLoS One. 2013;8:e56950
14. Ren YF, Li G, Wu J, Xue YF, Song YJ, Lv L. et al. Dicer-dependent biogenesis of small RNAs derived from 7SL RNA. PLoS One. 2012;7:e40705
15. Akopian D, Shen K, Zhang X, Shan SO. Signal recognition particle: an essential protein-targeting machine. Annu Rev Biochem. 2013;82:693-721
16. Tang KF, Ren H, Cao J, Zeng GL, Xie J, Chen M. et al. Decreased Dicer expression elicits DNA damage and up-regulation of MICA and MICB. J Cell Biol. 2008;182:233-9
17. Zhang PY, Li G, Deng ZJ, Liu LY, Chen L, Tang JZ. et al. Dicer interacts with SIRT7 and regulates H3K18 deacetylation in response to DNA damaging agents. Nucleic Acids Res. 2016;44:3629-42
18. Chen X, Li WF, Wu X, Zhang HC, Chen L, Zhang PY. et al. Dicer regulates non-homologous end joining and is associated with chemosensitivity in colon cancer patients. Carcinogenesis. 2017;38:873-82
19. Francia S, Michelini F, Saxena A, Tang D, de Hoon M, Anelli V. et al. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature. 2012;488:231-5
20. Wei W, Ba Z, Gao M, Wu Y, Ma Y, Amiard S. et al. A role for small RNAs in DNA double-strand break repair. Cell. 2012;149:101-12
21. Chitale S, Richly H. DICER- and MMSET-catalyzed H4K20me2 recruits the nucleotide excision repair factor XPA to DNA damage sites. J Cell Biol. 2018;217:527-40
22. Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186-90
23. Dhar P, Wu JD. NKG2D and its ligands in cancer. Curr Opin Immunol. 2018;51:55-61
24. Paludan SR, Reinert LS, Hornung V. DNA-stimulated cell death: implications for host defence, inflammatory diseases and cancer. Nat Rev Immunol. 2019;19:141-53
25. Gasser S, Zhang WYL, Tan NYJ, Tripathi S, Suter MA, Chew ZH. et al. Sensing of dangerous DNA. Mech Ageing Dev. 2017;165:33-46
26. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759-71
27. Yoshikawa T, Otsuka M, Kishikawa T, Takata A, Ohno M, Shibata C. et al. Unique haploinsufficient role of the microRNA-processing molecule Dicer1 in a murine colitis-associated tumorigenesis model. PLoS One. 2013;8:e71969
28. Stratmann J, Wang CJ, Gnosa S, Wallin A, Hinselwood D, Sun XF. et al. Dicer and miRNA in relation to clinicopathological variables in colorectal cancer patients. BMC Cancer. 2011;11:345
29. Papachristou DJ, Korpetinou A, Giannopoulou E, Antonacopoulou AG, Papadaki H, Grivas P. et al. Expression of the ribonucleases Drosha, Dicer, and Ago2 in colorectal carcinomas. Virchows Arch. 2011;459:431-40
30. Choi CR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol. 2017;14:218-29
31. Ma D, Chen X, Zhang PY, Zhang H, Wei LJ, Hu S. et al. Upregulation of the ALDOA/DNA-PK/p53 pathway by dietary restriction suppresses tumor growth. Oncogene. 2018;37:1041-8
32. Pereira C, Gracio D, Teixeira JP, Magro F. Oxidative stress and DNA samage: implications in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2403-17
33. Dabrowska N, Wiczkowski A. Analytics of oxidative stress markers in the early diagnosis of oxygen DNA damage. Adv Clin Exp Med. 2017;26:155-66
34. Mateescu B, Batista L, Cardon M, Gruosso T, de Feraudy Y, Mariani O. et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med. 2011;17:1627-35
35. Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773-89
36. Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T. et al. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68:10094-104
37. Jones MF, Hara T, Francis P, Li XL, Bilke S, Zhu Y. et al. The CDX1-microRNA-215 axis regulates colorectal cancer stem cell differentiation. Proc Natl Acad Sci U S A. 2015;112:E1550-8
38. Khella HW, Bakhet M, Allo G, Jewett MA, Girgis AH, Latif A. et al. miR-192, miR-194 and miR-215: a convergent microRNA network suppressing tumor progression in renal cell carcinoma. Carcinogenesis. 2013;34:2231-9
39. Zang Y, Wang T, Pan J, Wu R, Ge H, Qu B. et al. miR-215 promotes cell migration and invasion of gastric cancer cell lines by targeting FOXO1. Neoplasma. 2017 64
40. Deng Y, Huang Z, Xu Y, Jin J, Zhuo W, Zhang C. et al. MiR-215 modulates gastric cancer cell proliferation by targeting RB1. Cancer Lett. 2014;342:27-35
41. Karaayvaz M, Pal T, Song B, Zhang C, Georgakopoulos P, Mehmood S. et al. Prognostic significance of miR-215 in colon cancer. Clin Colorectal Cancer. 2011;10:340-7
42. Martin M, Lopez-Tarruella S, Gilarranz YJ. Endocrine therapy for hormone treatment-naive advanced breast cancer. Breast. 2016;28:161-6
43. Cicero AF, Baggioni A. Berberine and its role in chronic disease. Adv Exp Med Biol. 2016;928:27-45
44. Cazzaniga M, Bonanni B. Relationship between metabolic disorders and breast cancer incidence and outcomes. is there a preventive and therapeutic role for berberine? Anticancer Res. 2018;38:4393-402
45. Choi DS, Kim SJ, Jung MY. Inhibitory activity of berberine on DNA strand cleavage induced by hydrogen peroxide and cytochrome c. Biosci Biotechnol Biochem. 2001;65:452-5
46. Hou D, Xu G, Zhang C, Li B, Qin J, Hao X. et al. Berberine induces oxidative DNA damage and impairs homologous recombination repair in ovarian cancer cells to confer increased sensitivity to PARP inhibition. Cell Death Dis. 2017;8:e3070
47. Akyol-Salman I, Lece-Sertoz D, Baykal O. Topical pranoprofen 0.1% is as effective anti-inflammatory and analgesic agent as diclofenac sodium 0.1% after strabismus surgery. J Ocul Pharmacol Ther. 2007;23:280-3
48. Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y. et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256-64
49. Shirai T, Mori M, Uotani T, Chida K. Gastrointestinal disorders in anaphylaxis. Intern Med. 2007;46:315-6
Author Biography
 Dr. Kai-Fu Tang is the head of Digestive Cancer Center, the First Affiliated Hospital of Wenzhou Medical University. He has published more than 30 papers in international journals such as Genome Biol, J Cell Biol, Nucleic Acids Res, Oncogene, Pharmacol Res, Cell Death Dis, Carcinogenesis, J Biol Chem. The current research in Dr. Tang's laboratory is focused on the role of Dicer in carcinogenesis. Dr. Tang found that decreased Dicer expression promotes the development of inflammation-associated cancers via the following mechanisms. First, Dicer is essential for DNA repair; decreased Dicer expression causes DNA damage accumulation, which induce inflammation and gene mutation and eventually promote carcinogenesis. Second, Dicer processes 7SL RNA into small fragments, which function as dominant-negative regulators of the full-length 7SL RNA and interfere with signal recognition particle (SRP) complex formation. Decreased Dicer expression promotes SRP complex formation by down-regulating 7SL RNA fragments. This, consequently, enhances SRP-mediated protein targeting and increases the expression of membrane proteins and secretory proteins, which are up-regulated in tumor tissues. Finally, decreased Dicer expression may promote carcinogenesis via deregulating miRNA expression. Moreover, Dr. Tang found that decreased Dicer expression can enhance the efficiency of chemotherapy and radiotherapy; increased Dicer expression can alleviate inflammation and prevent inflammation-associated tumorigenesis. The ongoing work in Dr. Tang's laboratory is dedicated to identifying compounds that can either increase or decrease the expression and activity of Dicer and to investigating whether these compounds can be used for cancer treatment and/or prevention.
Dr. Kai-Fu Tang is the head of Digestive Cancer Center, the First Affiliated Hospital of Wenzhou Medical University. He has published more than 30 papers in international journals such as Genome Biol, J Cell Biol, Nucleic Acids Res, Oncogene, Pharmacol Res, Cell Death Dis, Carcinogenesis, J Biol Chem. The current research in Dr. Tang's laboratory is focused on the role of Dicer in carcinogenesis. Dr. Tang found that decreased Dicer expression promotes the development of inflammation-associated cancers via the following mechanisms. First, Dicer is essential for DNA repair; decreased Dicer expression causes DNA damage accumulation, which induce inflammation and gene mutation and eventually promote carcinogenesis. Second, Dicer processes 7SL RNA into small fragments, which function as dominant-negative regulators of the full-length 7SL RNA and interfere with signal recognition particle (SRP) complex formation. Decreased Dicer expression promotes SRP complex formation by down-regulating 7SL RNA fragments. This, consequently, enhances SRP-mediated protein targeting and increases the expression of membrane proteins and secretory proteins, which are up-regulated in tumor tissues. Finally, decreased Dicer expression may promote carcinogenesis via deregulating miRNA expression. Moreover, Dr. Tang found that decreased Dicer expression can enhance the efficiency of chemotherapy and radiotherapy; increased Dicer expression can alleviate inflammation and prevent inflammation-associated tumorigenesis. The ongoing work in Dr. Tang's laboratory is dedicated to identifying compounds that can either increase or decrease the expression and activity of Dicer and to investigating whether these compounds can be used for cancer treatment and/or prevention.
![]() Corresponding author: Kai-Fu Tang, M.D., Ph.D. Digestive Cancer Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, Zhejiang, P.R. China. Tel: 0577-88831271; Fax: 0577-88831359; Email: tang_kaifuedu.cn or tang_kaifucom
Corresponding author: Kai-Fu Tang, M.D., Ph.D. Digestive Cancer Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325015, Zhejiang, P.R. China. Tel: 0577-88831271; Fax: 0577-88831359; Email: tang_kaifuedu.cn or tang_kaifucom
 Global reach, higher impact
Global reach, higher impact