13.3
Impact Factor
Theranostics 2020; 10(14):6310-6321. doi:10.7150/thno.42573 This issue Cite
Research Paper
Phage-delivered sensitisation with subsequent antibiotic treatment reveals sustained effect against antimicrobial resistant bacteria
1. Academy of Military Medical Sciences, 27 Taiping Road, Haidian District, Beijing 100850, China
2. The Centre for Infectious Disease Control, Chinese PLA Centre for Disease Control and Prevention, 20 Dongda Street, Fengtai District, Beijing 100071, China
3. State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology, 20 Dongda Street, Fengtai District, Beijing 100071, China
4. Beijing Advanced Innovation Center for Soft Matter Science and Engineering (BAIC-SM), College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China
†These authors contributed equally to this article.
Abstract
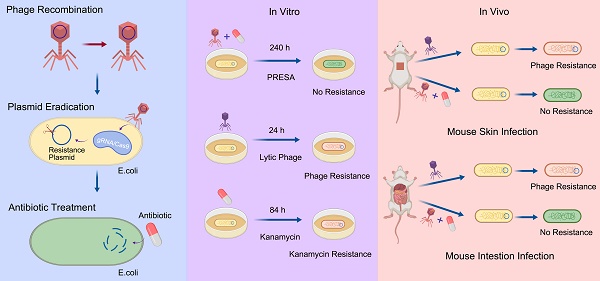
Temperate phages integrated with clustered regularly interspaced short palindromic repeat (CRISPR)/Cas systems have been gaining attention as potential strategies for combating bacteria resistant to antimicrobials. To further advance this technology, phage recombination procedure should be improved, and the bactericidal effect should be examined in detail and compared with conventional lytic phage strategy. The possibility of the emergence of mutational resistance, a phenomenon commonly observed with lytic phage therapy, should be illustrated.
Methods: Here, we developed a novel one-step cloning method to fulfil the recombination of CRISPR/Cas9 system within the genome of a new isolated lysogenic Escherichia coli phage. Then, we proposed and developed a phage-delivered resistance eradication with subsequent antibiotic treatment (PRESA) strategy. The removal efficiency and antimicrobial effect of the plasmids were analysed. Long-term antimicrobial effect was evaluated by continued OD600 monitoring for 240 hours to illustrate the potential mutational resistance, compared with the lytic phage strategy. The treatment effect of PRESA was evaluated in vivo by determining bacterial loads in the skin and intestine of infected mice, in contrast with lytic phage therapy. Genome sequencing was performed to identify mutations in bacterial cells treated with phage strategies.
Results: Phage-delivered CRISPR targeting efficiently eradicated and blocked the transfer of the antibiotic resistance plasmid. PRESA decreased the bacterial load by over 6- and 5-logs in vitro and in vivo, respectively. Importantly, while lytic phages induced mutational phage resistance at 24 h in vitro and 48 hours in vivo, PRESA demonstrated a constant effect and revealed no resistant mutants. Genes involved in DNA mismatch repair were upregulated in cells undergoing Cas9-based plasmid cleavage, which may reduce the development of mutations.
Conclusion: The PRESA strategy for eradicating resistant bacteria showed high bactericidal efficacy and a sustained inhibition effect against resistant bacteria. By restoring the efficacy of low-cost antibiotics, PRESA could be developed as an efficient and economical therapy for infections of antibiotic resistant bacteria.
Keywords: Antimicrobial resistance, Phage delivery system, CRISPR/Cas, Phage genome recombination, Phage therapy
 Global reach, higher impact
Global reach, higher impact