13.3
Impact Factor
Theranostics 2020; 10(15):6638-6660. doi:10.7150/thno.44793 This issue Cite
Research Paper
Genetic and pharmacological activation of Hedgehog signaling inhibits osteoclastogenesis and attenuates titanium particle-induced osteolysis partly through suppressing the JNK/c-Fos-NFATc1 cascade
1. Department of Orthopaedics, the First Affiliated Hospital of Soochow University, Suzhou, Jiangsu 215006, China
2. Orthopedic Institute, Medical College, Soochow University, Suzhou, Jiangsu 215007, China
3. Orthopedic Department, Taizhou Hospital Affiliated to Wenzhou Medical University, Linhai, Zhejiang 317000, China
4. Institute of Life Sciences, College of Life and Environmental Science, Key Laboratory of Mammalian Organogenesis and Regeneration, Hangzhou Normal University, Hangzhou, Zhejiang 310036, China
#These authors contributed equally to this study.
Abstract
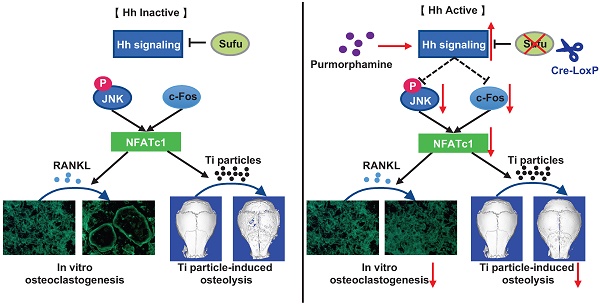
Rationale: Wear particle-induced periprosthetic osteolysis (PPO) is a common long-term complication of total joint arthroplasty, and represents the major cause of aseptic loosening and subsequent implant failure. Previous studies have identified the central role of osteoclast-mediated bone resorption in the pathogenesis of PPO. Thus, therapeutic approaches of inhibiting osteoclast formation and activity are considered to be of great potential to prevent and treat this osteolytic disease. Hedgehog (Hh) signaling has been shown to play an important role in promoting osteoblast differentiation and bone formation. While Hh signaling is also implicated in regulating osteoclastogenesis, whether it can directly inhibit osteoclast differentiation and bone resorption remains controversial. Moreover, its potential therapeutic effects on PPO have never been assessed. In this study, we explored the cell-autonomous role of Hh signaling in regulating osteoclastogenesis and its therapeutic potential in preventing wear particle-induced osteolysis.
Methods: Hh signaling was activated in macrophages by genetically ablating Sufu in these cells using LysM-Cre or by treating them with purmorphamine (PM), a pharmacological activator of Smoothened (Smo). In vitro cell-autonomous effects of Hh pathway activation on RANKL-induced osteoclast differentiation and activity were evaluated by TRAP staining, phalloidin staining, qPCR analyses, and bone resorption assays. In vivo evaluation of its therapeutic efficacy against PPO was performed in a murine calvarial model of titanium particle-induced osteolysis by μCT and histological analyses. Mechanistic details were explored in RANKL-treated macrophages through Western blot analyses.
Results: We found that Sufu deletion or PM treatment potently activated Hh signaling in macrophages, and strongly inhibited RANKL-induced TRAP+ osteoclast production, F-actin ring formation, osteoclast-specific gene expression, and osteoclast activity in vitro. Furthermore, we found that Sufu deletion or PM administration significantly attenuated titanium particle-induced osteoclast formation and bone loss in vivo. Our mechanistic study revealed that activation of Hh signaling suppressed RANKL-induced activation of JNK pathway and downregulated protein levels of two key osteoclastic transcriptional factors, c-Fos and its downstream target NFATc1.
Conclusions: Both genetic and pharmacological activation of Hh signaling can cell-autonomously inhibit RANKL-induced osteoclast differentiation and activity in vitro and protect against titanium particle-induced osteolysis in vivo. Mechanistically, Hh signaling hinders osteoclastogenesis partly through suppressing the JNK/c-Fos-NFATc1 cascade. Thus, Hh signaling may serve as a promising therapeutic target for the prevention and treatment of PPO and other osteolytic diseases.
Keywords: peri-prosthetic osteolysis, titanium particle, Hh signaling, osteoclatogenesis
 Global reach, higher impact
Global reach, higher impact