13.3
Impact Factor
Theranostics 2020; 10(15):6946-6958. doi:10.7150/thno.38236 This issue Cite
Research Paper
Preclinical evaluation of an 111In/225Ac theranostic targeting transformed MUC1 for triple negative breast cancer
1. Invicro, LLC, Boston, MA, USA.
2. Department of Biological Sciences, University of North Carolina, Charlotte, NC, USA.
3. OncoTAb, Inc., Charlotte, NC, USA.
*Equally contributing senior authorship.
Abstract
Rationale: Transformed MUC1 (tMUC1) is a cancer-associated antigen that is overexpressed in >90% of triple-negative breast cancers (TNBC), a highly metastatic and aggressive subtype of breast cancer. TAB004, a murine antibody targeting tMUC1, has shown efficacy for the targeted delivery of therapeutics to cancer cells. Our aim was to evaluate humanized TAB004 (hTAB004) as a potential theranostic for TNBC.
Methods: The internalization of hTAB004 in tMUC1 expressing HCC70 cells was assessed via fluorescent microscopy. hTAB004 was DOTA-conjugated and radiolabeled with Indium-111 or Actinium-225 and tested for stability and tMUC1 binding (ELISA, flow cytometry). Lastly, in vivo biodistribution (SPECT-CT), dosimetry, and efficacy of hTAB004 were evaluated using a TNBC orthotopic mouse model.
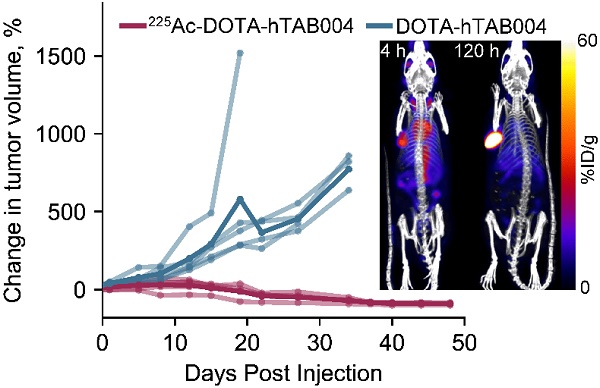
Results: hTAB004 was shown to bind and internalize into tMUC1-expressing cells. A production method of 225Ac-DOTA-hTAB004 (yield>97%, RCP>97% SA=5 kBq/µg) and 111In-DOTA-hTAB004 (yield>70%, RCP>99%, SA=884 kBq/µg) was developed. The labeled molecules retained their affinity to tMUC1 and were stable in formulation and mouse serum. In NSG female mice bearing orthotopic HCC70 xenografts, the in vivo tumor concentration of 111In-DOTA-hTAB004 was 65 ± 15 %ID/g (120 h post injection). A single 225Ac-DOTA-hTAB004 dose (18.5 kBq) caused a significant reduction in tumor volume (P<0.001, day 22) and increased survival compared to controls (P<0.007). The human dosimetry results were comparable to other clinically used agents.
Conclusion: The results obtained with hTAB004 suggest that the 111In/225Ac-DOTA-hTAB004 combination has significant potential as a theranostic strategy in TNBC and merits further development toward clinical translation.
Keywords: MUC1, Targeted Alpha Radiotherapy, Radioimmunotherapy, Theranostic
 Global reach, higher impact
Global reach, higher impact