13.3
Impact Factor
Theranostics 2020; 10(16):7150-7162. doi:10.7150/thno.47649 This issue Cite
Review
Primer design for quantitative real-time PCR for the emerging Coronavirus SARS-CoV-2
1. National Center for Clinical Laboratories, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, People's Republic of China.
2. Graduate School, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, People's Republic of China.
3. Beijing Engineering Research Center of Laboratory Medicine, Beijing, People's Republic of China.
#These authors contributed equally to this manuscript.
Abstract
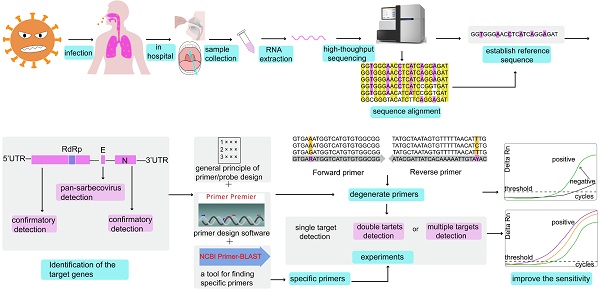
In December 2019, a new coronavirus disease (COVID-19) outbreak occurred in Wuhan, China. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), which is the seventh coronavirus known to infect humans, is highly contagious and has rapidly expanded worldwide since its discovery. Quantitative nucleic acid testing has become the gold standard for diagnosis and guiding clinical decisions regarding the use of antiviral therapy. However, the RT-qPCR assays targeting SARS-CoV-2 have a number of challenges, especially in terms of primer design. Primers are the pivotal components of a RT-qPCR assay. Once virus mutation and recombination occur, it is difficult to effectively diagnose viral infection by existing RT-qPCR primers. Some primers and probes have also been made available on the WHO website for reference. However, no previous review has systematically compared the previously reported primers and probes and described how to design new primers in the event of a new coronavirus infection. This review focuses on how primers and probes can be designed methodically and rationally, and how the sensitivity and specificity of the detection process can be improved. This brief review will be useful for the accurate diagnosis and timely treatment of the new coronavirus pneumonia.
Keywords: coronavirus, SARS-CoV-2, quantitative nucleic acid testing, primer design, sensitivity
 Global reach, higher impact
Global reach, higher impact