13.3
Impact Factor
Theranostics 2020; 10(16):7422-7435. doi:10.7150/thno.42167 This issue Cite
Review
Current understanding of the role of Adipose-derived Extracellular Vesicles in Metabolic Homeostasis and Diseases: Communication from the distance between cells/tissues
1. Department of Endocrinology, Health Management Center, Tianjin Union Medical Center, Nankai University Affiliated Hospital, Tianjin, 300121, P.R. China.
2. Department of Dermatology, Perelman School of Medicine, University of Pennsylvania.
3. Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA 19104, USA.
4. Shanghai National Research Centre for Endocrine and Metabolic Diseases, State Key Laboratory of Medical Genomics, Shanghai Institute for Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
#These authors contributed equally to this review.
Received 2019-11-17; Accepted 2020-5-26; Published 2020-6-12
Abstract
Extracellular vesicles (EVs) including exosomes, microvesicles (MVs), and apoptotic bodies, are small membrane vesicular structures that are released during cell activation, senescence, or programmed cell death, including apoptosis, necroptosis, and pyroptosis. EVs serve as novel mediators for long-distance cell-to-cell communications and can transfer various bioactive molecules, such as encapsulated cytokines and genetic information from their parental cells to distant target cells. In the context of obesity, adipocyte-derived EVs are implicated in metabolic homeostasis serving as novel adipokines. In particular, EVs released from brown adipose tissue or adipose-derived stem cells may help control the remolding of white adipose tissue towards browning and maintaining metabolic homeostasis. Interestingly, EVs may even serve as mediators for the transmission of metabolic dysfunction across generations. Also, EVs have been recognized as novel modulators in various metabolic disorders, including insulin resistance, diabetes mellitus, and non-alcoholic fatty liver disease. In this review, we summarize the latest progress from basic and translational studies regarding the novel effects of EVs on metabolic diseases. We also discuss EV-mediated cross-talk between adipose tissue and other organs/tissues that are relevant to obesity and metabolic diseases, as well as the relevant mechanisms, providing insight into the development of new therapeutic strategies in obesity and metabolic diseases.
Keywords: Extracellular vesicles, inflammation, adipose tissue, obesity, metabolic disease
Introduction
The prevalence of obesity has increased dramatically around the world [1]. It is estimated that more than 1.9 billion adults are overweight, of which over 650 million are obese [2]. Obesity is a major problem in children with 41 million children under the age of 5 and over 340 million children and adolescents aged 5-19 reported to be overweight or obese in 2016 [2]. If the current trend continues, the number of overweight or obese young children is expected to reach 70 million by 2025 [3]. Obesity has become a serious public health concern in the 21st century due to the rapid increase in its prevalence and the negative impact of its complications on human health [4]. Excess fat accumulation usually leads to various metabolic disorders, including insulin resistance (IR), type 2 diabetes mellitus (T2DM), and non-alcoholic fatty liver disease (NAFLD), resulting in a significant decrease in life expectancy as well as the quality of life [5, 6].
Adipose tissue (AT) was initially regarded as a type of tissue storing excess nutrients. However, recent studies demonstrated that AT could function as an endocrine organ, which secretes various adipokines, such as leptin, adiponectin, visfatin, resistin, and adipsin [7-9]. These AT-derived adipokines can serve as mediators to regulate the function of other metabolic organs [7-9]. Besides the above soluble mediators, extracellular vesicles (EVs), the subcellular membrane structures, have been shown to regulate pathophysiological conditions of other metabolic organs as insoluble mediators [10-12]. EVs derived from the adipose tissue (AT-derived EVs) are distinct from traditional soluble adipokines and can modulate specific target cells because of their bio-active cargos [13]. The role of EVs in human metabolic physiology and pathology has attracted increasing attention in the past few years. Given the involvement of EVs in various metabolic diseases, emerging information in this filed may provide insights into the development of potential new therapeutic strategies in obesity-related human diseases. In this review article, we will summarize recent advances from basic and translational studies of EVs and focus on their role in obesity and metabolic diseases.
Extracellular Vesicles
EVs, membranous subcellular structures with lipid bilayers and cytoplasmic components, are released from their parental cells in a highly regulated manner [11, 12]. The EV-associated bioactive cargos include proteins, lipids, multi-molecular complexes, and nucleic acids (DNA, RNA, siRNA, microRNA, and lncRNA), many of which have been shown to modulate gene expression and signaling pathways in target cells. EVs can be released from almost all types of cells, including normal, malignant, and senescent cells, and particularly cells undergoing several types of programmed cell death, like apoptosis, pyroptosis, and necroptosis [14-15]. EVs exist in various solid organs/tissues and biological fluids, such as urine, blood, breast milk, semen, and amniotic fluid [16], and are involved in physiological functions. In pathologic conditions, such as cancer, infectious, and metabolic diseases, elevated levels of EVs have been observed [17-19]. Based on the differences in size and biogenesis, EVs can be broadly categorized into exosomes, microvesicles (MVs), and apoptotic bodies [20]. Various EV isolation methods have been developed by exploiting the differences in size, buoyancy, or surface membrane marker expression [12].
Exosomes, the smallest EVs with the size range of 30 - 100 nm in diameter, are generated from the endosomal compartment and released into the extracellular space as nano-sized membrane vesicles. Pioneering studies from Harding et al. and Pan et al. suggested that exosomes arise by budding from the intracellular endosomal membranes [21-23]. At least three molecular mechanisms have been identified that are involved in the assembly and loading of EVs, like the endosomal sorting complex required for transport (ESCRT) machinery, sphingolipid ceramide, and tetraspanin CD63 [24, 25]. These mechanisms may regulate different bio-active cargos packed in diverse exosomes from a variety of cell types under various pathophysiological conditions. Most exosomes contain abundant lipids, including cholesterol, sphingolipids, and probably phosphatidylserine (PS) [26]. Further investigations of the molecular architecture and the underlying mechanisms in the assembly and loading of EVs are needed.
MVs (also called microparticles), ranging from 100 to 1,000 nm in diameter, are produced from the cell surface plasma membrane by a budding process. MVs, first described in 1967 by Peter Wolf, originated from platelets for their prothrombotic function [27]. Exposure to phosphatidylserine (PS) from the inner surface of the cell membrane is a common feature of MVs from activated or apoptotic cells [10, 11, 28]. MVs carry bioactive molecules from the membrane, cytoplasm, nucleus, and other organelles [10, 11]. Our previous studies have reported that cell membrane MMP-14, ADAM10/17, or nuclear HMGB1 could be released with MVs from human macrophages or neutrophils when exposed to tobacco smoke extract probably through apoptosis induction [29, 30]. In addition, EVs have recently been demonstrated to be derived from cells undergoing other types of programmed cell death, such as necroptosis and pyroptosis [13, 31].
In necroptic cells, EVs release is regulated by activation of receptor-interacting protein kinase-3 (RIPK3) and phosphorylation of mixed lineage kinase domain-like (MLKL) protein [32]. Like apoptotic cells, necroptotic cells also externalize PS on the outer plasma membrane after the membrane translocation of phospho-MLKL [32], which is enclosed and released with EVs, as a mechanism for the self-restricting action of cells from the necroptotic activity of MLKL [33]. In contrast, inhibition of MLKL phosphorylation could protect cells from necroptotic cell death, resulting in reduced EVs release and restricted inflammatory response [34]. Pyroptosis is a highly inflammatory form of programmed cell death, which is characterized by the release of interleukin-1β (IL-1β) or interleukin-18 (IL-18) [35]. Pyroptotic cells release these cytokines from cytoplasm through cell membrane pore molecule gasdermin D (GSDMD), the mature form of which is cleaved by caspase-1 and caspase 11/4/5 [36-40]. Pyroptotic cells release cytokine-containing MVs when encountered by various pathological stimulations, including stroke, heart attack, or cancer [41]. Furthermore, pyroptosis could also serve as a novel driver of the inflammatory response in liver injury and fibrosis [42].
These studies have demonstrated that EVs derived from apoptotic, necroptotic, and pyroptotic cells may have different functions in target cells, probably because of the differences in MV-associated bioactive cargos that are released by different mechanisms. The bioactive molecules of EVs may be involved in both physiological and pathological processes, and contribute to various human diseases, including obesity and metabolic diseases.
Besides programmed cell death, recent studies showed that MVs could also be released by senescent cells [15, 43]. Obese individuals have increased levels of circulating pro-inflammatory cytokines with age that might be secreted by senescent cells, leading to the development of metabolic diseases [44]. Targeting human senescent fat cell progenitors could suppress the release of activin A, an adipokine that regulates energy balance and insulin insensitivity [45]. Senescence-associated EV secretion from cancer cells was first described by Lehmann et al. [15]. So far, DNA-damaging reagents, irradiation, serial passaging, and oncogenic Ras expression have been shown to contribute to the secretion of EVs from senescent cells [46]. Additionally, harmful molecules produced during stress or pathological conditions could be eliminated from senescent cells by the release of EVs as “dumping garbage” to maintain cellular integrity and homeostasis [47]. Interestingly, the levels of extracellular eNAMPT (nicotinamide phosphoribosyltransferase) declined with age in mice and humans, while supplementing eNAMPT-containing EVs could improve physical activity and extend mouse life span [48]. Furthermore, the impact of gut microbiota on human health has recently attracted much attention that may involve membrane vesicles. Shen et al. reported that administration of outer membrane vesicles (OMVs) isolated from Bacteroides fragilis could deliver commensal molecules, which mimic the benefits of microbiota [49]. This finding was confirmed by other studies in which bacteria-derived OMVs have been shown to effectively modulate host responses, and activate signaling events through the intestinal epithelial barrier [50].
In summary, in response to various stimuli, EVs could be released by various cell types as well as bacteria to modulate the function of near or distant cells. EVs provide an alternative mode of paracrine and endocrine communications compared to the conventional chemical signaling for intercellular communications including direct cell-cell contact or receptor-mediated recognition of soluble hormones and cytokines [43]. It is still unclear if EVs can serve as a specialized messaging system in the body. However, specific molecules on the surface membrane of EVs may serve as a special “bar code” recognized by their distantly located special receptors. A recent study reported that cytokines present on the EV surface could target distant recipient cells that express appropriate cytokine receptors [43]. Taken together, the direct interaction between EV surface molecules and receptors on the target cells allows EVs to specifically interact with target cells. Although there are still many unanswered questions that need to be addressed, a growing body of research on EVs is expected to generate new information that would provide a better understanding of this field.
EVs and Adipose Tissues
Adipose-derived EVs serve as novel adipokines
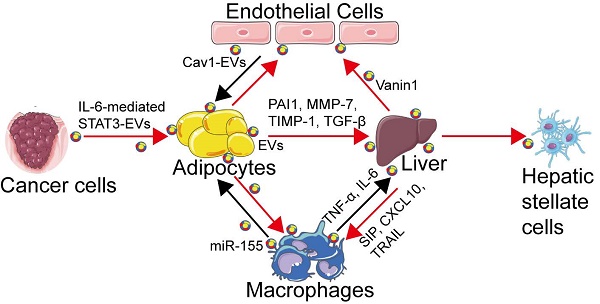
Clinical studies have shown that gastric bypass surgery and the subsequent weight loss can improve insulin resistance (IR) to maintain glucose homeostasis and this beneficial effect might be mediated by circulating adipocyte-derived exosomes [51]. AT-derived EVs have been reported to mediate the endocrine link between maternal AT and fetal growth, and to be responsible for fetal overgrowth [52]. Besides, AT-derived EVs under hypoxic conditions might promote lipid synthesis by increasing the levels of lipogenic enzymes, including fatty acid synthase (FASN), glucose-6-phosphate dehydrogenase (G6PD) and acetyl-CoA carboxylase (ACC), which may reflect metabolic stress in adipocytes [53]. These studies have suggested the potential role of EVs as adipokines contributing to adipose tissue homeostasis or dysfunction (Figure 1). Furthermore, AT-derived EVs can also aggravate IR by stimulating monocyte differentiation and macrophage activation, as well by releasing tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) [54]. Exosomes isolated from visceral adipose tissue (VAT) of obese patients could be integrated into hepatocytes resulting in dysregulation of transforming growth factor-beta (TGF-β) pathway, and promoting the development of NAFLD [55]. Besides, AT-derived EVs could also contribute to liver fibrogenesis by extracellular matrix (ECM) accumulation in the liver involving plasminogen activator inhibitor (PAI-1), matrix metalloproteinase (MMP)-7, and tissue inhibitors of metalloproteinase (TIMP)-1 [56, 57].
Thus, AT-derived EVs may serve as adipokines to modulate metabolic dysfunction through regulation of adipose tissue homeostasis, promotion of adipose inflammation, or interference with the normal signaling pathways of liver and occurrence of hepatic inflammation and even liver fibrosis. These studies may help us gain a better understanding of the role of AT-derived EVs in the cross-talk between AT and other metabolic organs in the context of obesity.
Brown adipose tissue (BAT)-derived EVs and white adipose tissue (WAT) browning
Adipose tissues can be classified as white adipose tissue (WAT) and brown adipose tissue (BAT). WAT stores excessive energy in the body, while BAT generates heat under cold stress (non-shivering thermogenesis) with the mediation of uncoupling protein 1 (UCP1), a transmembrane protein in the mitochondrial inner membrane in brown adipocytes [58]. The importance of BAT in human metabolism has been demonstrated and its amount was reported to be inversely correlated with body mass index [14]. Since mass and activity of BAT decrease with age, it is worthwhile to explore reversing this adverse progression. Remarkable findings from several groups showed that there is a continuum between BAT and WAT in rodents, in which cold exposure or stimulation with adrenergic agonist may be important for elevated UCP1 expression in WAT [59, 60]. Interestingly, BAT can secret EVs for communication with other metabolic organs. A recent study by Jung et al. reported that human adipose-derived stem cells (HASCs) could be differentiated into beige/brown adipocytes by EVs derived from HASCs generated during beige adipogenic differentiation [61]. Applying these EVs in vivo attenuated high-fat diet (HFD)-induced hepatic steatosis and improved glucose intolerance through browning of the adipose tissue in mice [61].
Adipose-derived EVs can function as novel adipokines. Under stimulation by adipocyte-derived EVs, monocytes can transform into activated macrophages with the induction of IR via releasing inflammatory cytokines (such as TNF-α, IL-6, TLR4/TRIF). EVs from WAT can act on the liver and lead to hepatic steatosis and fibrogenesis via involvement of TGF-β, PAI-1, MMP-7, and TIMP-1. EVs derived from adipocytes can impair endothelial function and promote the development of obesity-related metabolic diseases. EVs secreted by hypoxic adipocytes favor the expression of lipogenic enzymes (such as FASN, G6PD, and ACC) that can promote lipid synthesis.
Thomou et al. studied the role of circulating miRNAs in AT by generating mice with adipose tissues deficient in miRNA-processing enzyme Dicer (DicerKO) [62] and found that DicerKO mice exhibited lipodystrophy, BAT whitening, and IR, with reduced circulating miRNAs. Transplantation of wild-type BAT into DicerKO mice, on the other hand, could improve glucose tolerance and reduce fibroblast growth factor-21 (FGF21) in the liver and serum, in which EV-associated miRNAs from BAT might act as novel forms of adipokines to distantly regulate gene expression in the liver [62]. These novel findings suggest that, in addition to promoting energy expenditure, BAT-derived EVs also play a role in metabolism, i.e. WAT browning, and cross-talk with the distant organ, the liver [62]. Other investigations that analyzed the miRNA expression profile of human subcutaneous adipose tissue during adipocyte differentiation, suggested a close cross-talk between adipogenesis and miRNAs [63, 64].
Overall, the above promising studies indicate that EVs derived from beige/brown adipocytes have beneficial effects on WAT browning, thus providing insights into the development of potential therapeutic strategies for future treatment of obesity and metabolic disorders. Since many of the studies are from rodents, additional investigations in humans are necessary to determine the positive and negative functions of AT-derived EVs. Furthermore, adipogenic miRNAs may serve as biomarkers and therapeutic targets for obesity and its related complications [63, 64].
Interaction between EVs derived from other cell types and AT
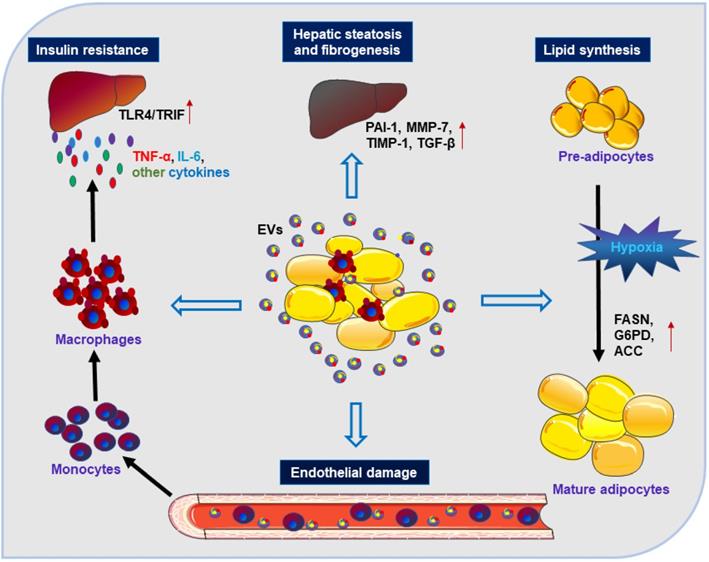
EVs derived from other cell types, such as leukocytes, erythrocytes, platelets, and hepatocytes, may also influence AT and its metabolism [65, 66]. A cross-sectional and longitudinal cohort study found that levels of erythrocyte-derived EVs were elevated in individuals with diabetes, and internalization of these diabetic EVs by leukocytes altered their function resulting in secretion of proinflammatory cytokines [67]. Furthermore, hepatic EVs were also metabolically active and were involved in oxidative stress, endothelial dysfunction, and drug-induced liver injury [68, 69]. Our earlier work demonstrated that tobacco smoke exposure could induce the release of potent collagenolytic MMP-14-containing EVs from cultured macrophages [29]. Subsequently, the macrophage-derived collagenolytic EVs were found to contribute to the expansion of AT through degradation of pericellular collagenous web around adipocytes [70]. These findings may help explain the abdominal obesity in smokers [70].
Caveolin 1 (cav1) is an important membrane-bound structural and signaling protein, which is highly abundant in adipocytes and endothelial cells (ECs) [69]. Interestingly, Crewe et al. found abundant cav1 protein expression in adipocytes even though it had been specifically ablated [71]. By using genetically engineered mouse models, these investigators found that ECs could transfer their released EVs with cav1 to adipocytes that reciprocated by releasing EVs to ECs [71]. These cav1-associated EVs were important for not only adipose homeostasis but also systemic metabolic state [71]. Taken together, the above studies demonstrated that EVs from non-adipose tissues, i.e. erythrocytes, macrophages, endothelial cells, or hepatocytes are important in whole-body adipose tissue homeostasis (Figure 2). Long distance communication between other organs/tissues and the adipose tissue may be mediated through secretion of EVs. However, there are still many questions that need to be addressed and the detailed mechanisms investigated.
Besides the role of EVs in adipose homeostasis, miRNA-containing EVs obtained from ATM of obese mice could induce glucose intolerance and IR when injected into lean mice [71]. Conversely, the administration of the ATM EVs isolated from lean mice could reverse the negative changes in obese recipients [71]. Our recent work demonstrated that tobacco smoke exposure of macrophages might release EVs with high mobility group box 1 (HMGB1) [72], which could directly impair insulin signaling in cultured adipocytes [12]. These results may help explain the adverse effects of tobacco smoking on insulin signaling impairment [73]. Furthermore, the overexpressed miR-155 in EVs from obese ATM suppressed gene expression of its downstream peroxisome proliferator-activated receptor γ (PPARγ) in insulin target organs, including AT, liver and muscle, through paracrine or endocrine mechanisms, leading to impaired insulin sensitivity and glucose homeostasis [74]. In contrast, EVs from ADSCs could trigger WAT browning and attenuate inflammation by induction of anti-inflammatory M2 macrophage polarization, alleviating obesity and hepatic steatosis with improved insulin sensitivity [75].
In summary, EVs, released from cells other than adipocytes, carry cargos including miRNAs and other bioactive proteins, and are involved in modulation of adipose homeostasis and metabolic states. These studies suggest that investigations on EVs provide insights into the understanding of metabolic systems for the development of novel therapeutic strategies and better treatments of metabolic diseases.
Impacts of EVs derived from other cell types on adipocytes. Exposure of ATM to stimuli (such as tobacco smoke) can result in the release of EVs with HMGB1 and MMP14 that contribute to IR and expansion of the size and volume of adipocytes. EVs from obese ATM harbor miR-155 and can impair insulin signaling and metabolic homeostasis through inhibition of PPARγ gene expression in AT. Endothelial cells stimulated by glucagon can transfer EV-associated cav1 into adipocytes to modulate adipose homeostasis. EVs released from cancer cells can promote WAT browning and lipolysis via activation of the IL-6/STAT3 signaling pathway.
EVs serve as mediators for intergenerational transmission of metabolic disease risk
Numerous studies have demonstrated that paternal/maternal metabolic disease risk can be transmitted from parents to offspring [76-79]. Although genomic DNA transmits inheritance, recent studies demonstrated that epigenetic information also contributes to the non-genetic intergenerational transmission of disease risk to future generations [80, 81]. Chen and co-workers reported that injection of sperm tRNA-derived small RNAs (tsRNAs) from male mice with HFD into normal zygotes generated metabolic disorders with altered gene expression of metabolic pathways in early embryos and islets of F1 offspring [82]. This novel observation was confirmed by a later study [83], and the changes were independent of DNA methylation at CpG-enriched regions [81, 82]. Another study showed that the tsRNAs were transferred to the sperm via epididymal EVs [84]. Thus, the evidence suggested that EVs contribute to the intergenerational transmission of the metabolic disease risk from paternal/maternal exposures to their future generations [84-86] and function as mediators of intracellular communication in the mammalian reproductive system [87-90]. The studies also indicated that EVs enable external factors from environmental exposure to reach gametes or zygote, as well as the tissues of the maternal reproductive tract, tracking parental exposure for the offspring [87-90].
Chan et al. reported that reproductive tract EVs could transmit information regarding stress in the paternal environment to sperm, potentially altering fetal development [89], and the changes in protein and miRNA content of EVs were long-lasting, suggesting a sustainable programmatic change in response to chronic stress [89]. Thus, in sperm maturation, EVs can perform a role in the intergenerational transmission of paternal environmental experience, providing direct communication between somatic cells and germ cells [88, 89, 91]. However, these observations raise an interesting question regarding the mechanism of EVs' involvement in the intergenerational transmission of paternal environmental experience? Prevailing data suggest that EVs transfer proteins, lipids, and small RNAs from the epididymal fluid to sperm, promoting sperm motility and oocyte recognition [92]. Importantly, the EV cargos, especially small RNAs, exhibit dramatic changes in response to environmental conditions such as smoking, drugs, or dietary constraints, and these environmental signals of paternal experience can be dynamically transferred to sperms by EVs [93-95]. Chan et al. reported that alterations of miRNA and protein content of epididymal epithelial cell-derived EVs in response to chronic stress were transferred to sperm transmitting the information from paternal cells to offspring [89]. Other small noncoding RNAs could also be transferred to sperm by epididymal EVs. The tsRNAs accounted for 80% of the small RNA content of sperm in the cauda epididymis as per small RNA-sequencing [84]. As the levels of the specific tsRNAs were affected in the zygote by low protein diet, the gene expression related to a metabolic phenotype was also altered in the offspring of the protein-restricted males [84].
Collectively, these novel findings support the role of EVs as a vector in transmitting paternal environmental exposure (for eg. HFD [84]) and encoding these experiences to sperm for delivery to the offspring [84,89]. These studies helped us understand the intergenerational transmission of metabolic disease risks, and may provide insights into the development of new treatments.
EVs and metabolic diseases
EVs are known to be involved in various human diseases, including obesity and metabolic disorders. Circulating EVs and EV-associated bioactive molecules, including miRNAs, reflect the characteristics of the parental cells and are ubiquitously present in a variety of human biofluids. Therefore, EVs have been proposed to be novel diagnostic and prognostic biomarkers in metabolic diseases [96]. One such example is of Perilipin A present in circulating EVs derived from adipocytes that represented a novel biomarker of AT stress [97]. Other studies have reported elevated levels of circulating EVs in obesity and its related metabolic disorders, i.e. IR, diabetes, and NAFLD [98-100]. Given the clinical relevance and pathological involvement of EVs in IR, diabetes, and NAFLD, we provide a more detailed discussion of EVs in these metabolic diseases in the following.
EVs and IR
As a major feature of T2DM, IR caused by impaired insulin signaling pathway is relevant to the development of hypertension and atherosclerosis. Freeman and co-workers recently reported higher plasma EV concentrations in patients with DM and its positive association with homeostasis model assessment (HOMA)-IR [67]. They found several insulin-signaling proteins in the circulating EVs, indicating that IR may contribute to higher plasma EV levels [67]. In contrast, EVs originating from different tissues/organs, including AT and muscle, may be involved in the development of IR [101]. EVs isolated from insulin resistant mice could modulate insulin signaling in pancreatic β-cells [102] or skeletal muscle [103], suggesting a role for EVs in insulin signaling in mice.
EVs derived from human subcutaneous or visceral fat tissue may impair insulin signaling in hepatocytes by inhibiting insulin-induced Akt phosphorylation, thus contributing to systemic IR development [104, 105]. Yu et al. reported that adipocyte-derived exosomal miR-27a may induce IR in skeletal muscle by suppressing PPARγ expression [104]. These studies demonstrated the potential role of adipocyte-derived EVs in IR development by cross-talking between adipose tissue and insulin sensitive organs, like liver and skeletal muscle [102, 104-107]. Interestingly, EVs released from hypoxic adipocytes and obese individuals can impair insulin-stimulated uptake of 2-deoxyglucose in adipocytes, suggesting that EVs act as mediators for transferring hypoxia-induced IR signatures within the adipose tissue [106]. Furthermore, adipocyte-derived EVs also lead to IR by down-regulating expression of insulin receptor substrate-1 (IRS-1) and hormone-sensitive lipase (HSL) in adipocytes [108]. In addition to the role of adipocyte-derived EVs, muscle EVs from mice with HFD-induced IR can integrate into the pancreas in vivo and modulate gene expressions in cultured β-cells and isolated islets in vitro, causing β-cell proliferation, thus explaining adaptations in beta cell mass during IR [102]. Consistent with this study, skeletal muscle has recently been shown to be an active endocrine organ that secretes myokines and contributes to the development of IR [107].
Obesity is characterized by chronic low-grade inflammation, which leads to the development of IR [109]. Adipocytes and AT-derived EVs may differentiate monocytes to a phenotype of AT macrophages and contribute to AT inflammation and IR [54]. Indeed, EVs from AT of HFD-induced mice when injected into control mice could increase the levels of inflammatory factors, including IL-6 and TNF-α, leading to the development of IR, via TLR4 pathway as this effect was attenuated in TLR4 KO mice [54]. A recent study demonstrated that EVs isolated from cultured 3T3-L1 adipocytes could induce pro-inflammatory M1 polarization of macrophages through activation of Ptch/PI3K signaling pathways by the EV-associated sonic hedgehog protein [110]. These results demonstrated that adipocyte-derived EVs can modulate IR development by affecting macrophage inflammatory responses. Conversely, macrophage-derived EVs can also contribute to the IR development through different mechanisms. Exosomal miR-155 secreted by AT macrophages of obese mice has been shown to impair Akt phosphorylation and repress PPARγ expression, thus aggravating IR in insulin target organs such as AT, skeletal muscle, and liver [74]. Our recent study demonstrated that macrophage-derived EVs carry HMGB1, a nuclear nonhistone DNA-binding protein that functions as a proinflammatory cytokine and can directly impair insulin signaling in cultured adipocytes in vitro [12]. We had previously reviewed the association between EVs and insulin sensitivity [19].
Hepatokines are liver-derived proteins, including fetuin A, fetuin B, retinol-binding protein 4 (RBP4) and selenoprotein P, which are associated with the induction of metabolic dysfunction [39, 61]. Contrary to the harmful effects of cytokines on IR and glucose dysregulation in obesity and NAFLD, recent studies indicated that type I interferon (IFN) may protect against metabolic dysfunction [108, 111]; other studies reported that IFNs are associated with EVs in various pathophysiological conditions [43, 112, 113]. Our laboratory recently provided evidence that EV-associated mitochondrial antiviral signaling (MAVS) triggered IFNβ production from dendritic cells [112]. However, it has not been evaluated if EV-associated IFNα or IFNβ can protect against metabolic dysfunction.
Overall, published literature underscored that EVs derived from adipocytes, skeletal muscles, or adipose tissue infiltrated macrophages and might contribute to IR development by interfering insulin signaling in insulin sensitive organ/tissues or causing pancreatic β-cell dysfunction. These results identify EVs as potential future therapeutic targets for the management of IR and metabolic syndromes.
EVs and type 1 diabetes mellitus
The appearance of diabetes can be attributed to the dysregulated crosstalk between endocrine organs, such as AT, liver, and skeletal muscles, that are involved in the development of metabolic diseases [54]. So far, we have discussed that EVs may play an important role in the pathogenesis of IR; here, we expand the discussion to the effects of EVs on the development of T2DM. Recent studies also suggested that EVs may contribute to the etiology of type 1 diabetes mellitus (T1DM) [113, 114].
T1DM is a disease characterized by defective insulin synthesis as a consequence of auto-immunologic injury of pancreatic β-cells, which accounts for 5-10% of patients with diabetes [115]. A growing body of literature has shown multiple roles of EV-associated miRNAs in T1DM [114, 116, 117]. A recent study by Guay and co-workers found that rodent and human T lymphocyte-derived EVs containing miR-142-3p, miR-142-5p, and miR-155 could be transferred to β cells resulting in apoptosis and contributing to the development of T1DM [117]. Furthermore, Rutman et al. demonstrated that EVs from human islets of Langerhan cells could activate B cells in T1DM patients to produce antibodies against glutamic acid decarboxylase 65 (GAD65), an early marker for beta cell destruction [116]. Interestingly, a recent study also demonstrated that the initial autoimmune response in T1DM was induced by exosomal β-cell autoantigens, such as GAD65 and IA-2 in response to endoplasmic reticulum stress [118]. Therefore, lymphocyte-derived EVs can induce β cell damage, while destructive islet cells can release GAD65-containing EVs that trigger B cells to produce GAD65 auto-antibody, indicating the role of EVs in the etiology and development of T1DM. Besides their pathogenic effects, EVs may also serve as biomarkers in T1DM. Lakhter et al. observed that cultured β-cells could release miR-21-5p with EVs under cytokine stimulation in vitro, and serum EV-derived miR-21-5p was increased threefold in children with new-onset T1DM compared with healthy children [119].The authors proposed that circulating EV-associated miR-21-5p may serve as a biomarker for the development of T1DM [119]. Another study assessed miRNAs expression in urinary EVs from individuals with T1DM and found enrichment of miR-130a and miR-145, while miR-155 and miR-424 were reduced [120]. These findings provide compelling evidence that EVs bearing miRNAs can be involved in the pathological process of T1DM, probably through transferring to β cells to cause apoptosis or induce autoimmune response. In addition, compared with healthy individuals, miRNAs in serum and urinary EVs from T1DM patients show different levels, which indicate EVs containing miRNAs may act as a novel marker of T1DM in the future.
EVs and type 2 diabetes mellitus
T2DM is characterized by relative insulin deficiency due to a progressive insufficiency in insulin secretion in individuals with IR, which accounts for 90-95% of patients with diabetes [104]. A meta-analysis of 48 studies demonstrated elevated circulating EVs derived from endothelium, platelets, and monocytes in T2DM patients as compared to those in controls [121], suggesting a potential role of EVs in the pathogenesis of T2DM or its complications. Fu and co-workers reported that hepatocellular EVs derived from HFD-induced obese mice promoted islet β cell compensatory hyperplasia in obesity and insulin resistance [122]. Jalabert et al. indicated that EVs released from HFD-induced, insulin-resistant muscles could cause downregulation of Ptch1, a negative regulator of Hedgehog signaling in pancreatic development [102]. Thus, either hepatocyte- or muscle-derived EVs may act distantly to influence the β-cell mass during the development of IR and T2DM [102, 122]. Also, various studies, including ours [123], have demonstrated that EV-associated molecules may impair insulin signaling in cultured adipocytes [11, 19, 72, 110, 123]. Isolated circulating exosomes, but not MVs, from patients with metabolic syndromes could also decrease insulin signaling in cultured hepatocytes [124].
Furthermore, several studies have demonstrated that EVs are associated with the pathogenesis of T2DM and contribute to the development of its related complications [125, 126]. Rossi et al. reported that water channel aquaporins (AQPs) 5 and 2 expressed on the plasma membrane of epithelial tubular cells were significantly increased in diabetic nephropathy (DN) patients as well as in the urine EVs from 35 diabetic patients [127]. Interestingly, urinary EV-associated AQP5 was also correlated with the histological grade of diabetic nephropathy (DN), thus may serve as novel noninvasive biomarkers in classifying the clinical stage of DN [127]. Interestingly, IR has been shown to cause diminished glucose uptake in similar regions of the brain in patients with Alzheimer's disease and T2DM. In this context, Kapogiannis et al. have shown that neural-derived blood EVs carry insulin receptor substrate 1 (IRS-1) in patients with preclinical Alzheimer's disease [128].
Collectively, EVs appear to exert crucial roles in T2DM development via interfering with pancreatic islet mass homeostasis, or modulating insulin signaling in adipose tissues or liver. EVs may also contribute to the complications of T2DM. Thus, EVs might be novel therapeutic targets for the treatment of IR and protection of β-cell dysfunction during the development of T2DM [101,114].
EVs and NAFLD
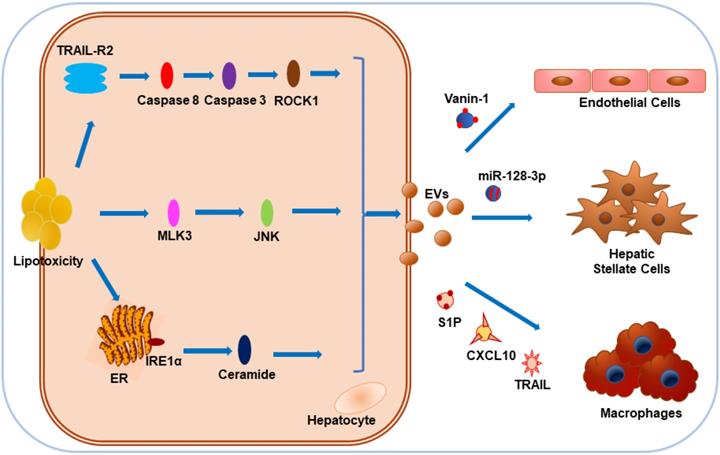
NAFLD encompasses a broad spectrum of conditions, including isolated hepatic steatosis, NASH, and cirrhosis [129]. With the accumulation of certain toxic lipids in cells, lipotoxicity-associated hepatocyte damage is considered as one of the key events that promote NAFLD progression [130]. Interestingly, toxic lipids including palmitate (PA) and lysophosphatidylcholine (LPC) could stimulate EVs release from hepatocytes of rodent animals and humans [122,131,132]. Recent publications suggest that EVs play an important role in the physiology and pathophysiology of liver diseases [133]. EVs derived from lipotoxic hepatocytes might promote hepatic inflammation, angiogenesis, and fibrosis as multiple-hit mechanisms of NAFLD pathogenesis [134]. EV release by LPC was mediated by Rho-associated, coiled-coil-containing protein kinase 1 (ROCK1) and tumor necrosis factor-like apoptosis-inducing ligand (TRAIL) receptor 2 (TRAIL-R2) signaling cascade, including TRAIL-R2, caspase 8 and caspase 3 [132]. Administration of ROCK1 inhibitor could reduce the release of hepatocyte-derived EVs containing TRAIL, leading to attenuated liver injury, inflammation, and fibrosis [132].
In NAFLD, the damaged hepatocytes induced the infiltration and activation of macrophages and other immune cells important for the development of inflammation [132, 134]. Ibrahim et al. found C-X-C motif chemokine 10 (CXCL10) as another protein cargo on LPC-induced EVs and proposed that MLK3 could induce lipotoxic hepatocytes to release CXCL10-enriched EVs, which were chemo-attractive toward macrophages in vitro, while MLK3-/- mice were protected against the development of dietary steatohepatitis [135, 136]. Hepatocytes cultured with PA could also release EVs enriched in C16:0 ceramide in an inositol-requiring enzyme 1α (IRE1α)-dependent manner and the macrophage chemotaxis could be activated by the ceramide metabolite, sphingosine-1-phosphate (S1P) [137]. Furthermore, Bruno et al. demonstrated that lipotoxic injury-induced EVs from hepatocytes could stimulate activation of M1 macrophages through EV-associated miR-192-5p, the blood levels of which positively correlated with hepatic inflammatory activity score and disease progression in NAFLD patients [138]. Interestingly, mice and patients with NASH showed high plasma levels of EV-associated mitochondrial DNA, which could selectively upregulate TNF-α through activation of TLR9, while removal of these EVs or treatment with TLR9 antagonist blocked the development of NASH [139]. Therefore, it is plausible that hepatocyte-derived EVs can contribute to the NAFLD through macrophage recruitment and activation, or induction of proinflammatory cytokines [135-139]. NAFLD progression is characterized by liver inflammation and fibrosis after repeated and sustained injuries, leading to end-stage liver diseases, such as cirrhosis and hepatocellular carcinoma.
Povero et al. found that hepatocyte-EV-associated miR-128-3p might be efficiently internalized by hepatic stellate cells (HSCs) in response to lipotoxicity, thus facilitating the development of liver fibrosis by repressing PPARγ expression [140]. Also, with the stimulation of lipid-induced toxicity, hepatocyte-derived EVs display pro-angiogenic features and are internalized by endothelial cells via vanin-1, a surface cargo protein [131]. To sum up, it appears that during lipotoxicity, various contents of EVs originating from hepatocytes, such as proteins, miRNAs, and mitochondrial DNA, exert a crucial impact on NAFLD progression by influencing hepatic macrophages, liver fibrosis, or angiogenesis. More research in a clinical setting is required to test this hypothesis (Figure 3).
Conclusions and Future Perspectives
Herein, we have summarized the recent literature depicting the role of adipocyte-derived EVs in several metabolic diseases. Numerous studies reported that WAT may contribute to metabolic disorders, while the browning WAT and BAT exerted beneficial effects. In recent years, EVs have attracted much attention for their role in metabolic dysfunction, in particular obesity and its complications. Studies have demonstrated that EVs derived from many cell types serve as novel mediators for long-distance communication between various cells and organs, and are involved in the development of obesity-associated metabolic disorders, including IR, diabetes, and NAFLD. Interestingly, EVs have been shown to transfer transgenerational information of metabolic disease risks from parents to the offspring in an epigenetic fashion, challenging the classical concept of genetic transmission across generations. Acting as novel mediators and biomarkers in the crosstalk between organs, EVs are crucial for maintaining metabolic homeostasis and regulating metabolic disorders. Investigation of the pathophysiology of EVs provides new opportunities in diagnosing and combatting metabolic disorders. However, there remain many outstanding challenges in the field. For example, the lack of specific, unambiguous EV markers and the dearth of adequate EV isolation methods represent serious limitations in the EV research field. Advanced investigations in analyzing the physical, chemical, and biological features of EVs may help further our understanding of EV functions and applying the relevant knowledge in clinical practice. Furthermore, the investigations of the role of EVs in the pathophysiologic processes of human diseases and the underlying molecular mechanisms are still preliminary. Last but not least, clinical research as well as applications of EVs in clinical practice is still limited. Therefore, further research in this field is warranted. Ongoing investigations are expected to provide insights into the role of EVs in metabolic homeostasis and disorders, and, in the future, afford powerful tools for the applications of EVs in diagnosis, treatment, and prognosis of metabolic diseases.
Impact of hepatocyte-derived EVs on NAFLD under lipotoxicity. Hepatocytes tend to release EVs in response to toxic lipids, including PA and LPC. Lipotoxicity-induced EV release is dependent on TRAIL-R2 signaling, stress kinase MLK3, and ER stress sensor IRE1α. CXCL10 and ceramide-bearing EVs mediate monocyte/macrophage chemotaxis to hepatocytes while TRAIL-laden EVs activate macrophages. Vanin-1-enriched EVs can mediate endothelial cell migration, while miR-128-3p-bearing EVs contribute to the proliferation and activation of HSCs.
Abbreviations
ACC: acetyl-CoA carboxylase; AdicerKO: knockout of the miRNA-processing enzyme Dicer; ADSCs: Adipose-derived stem cells; AQPs: Aquaporins; ATM: Adipose tissue macrophages; BAT: Brown adipose tissue; CAC: Cancer-related cachexia; Cav1: Caveolin 1; CCN2: Connective tissue growth factor; CD: Chow diet; CTLA4: Cytotoxic T-lymphocyte antigen 4; CXCL10: C-X-C motif chemokine 10; DAMPs: Danger-associated molecular patterns; DN: Diabetic nephropathy; ECs: Endothelial cells; ECM: Extracellular matrix; Enampt: Extracellular nicotinamide phosphoribosyltransferase; ESCRT: Endosomal Sorting Complex Required for Transport; EVs: Extracellular vesicles; FASN: fatty acid synthase; GAD65: glutamic acid decarboxylase 65; GC: gastric cancer; G6PD: glucose-6-phosphate dehydrogenase; GSDMD: gasdermin D; HFD: high-fat diet; HIF: hypoxia-inducible factor; HMGB1: high mobility group box 1; HOMA: homeostasis model assessment; HPA: hypothalamic-pituitary-adrenal; HSCs: hepatic stellate cells; HSL: hormone sensitive lipase; IL-18: interleukin-18; IL-1β: interleukin-1β; IL-6: interleukin-6; IR: insulin resistance; IRS-1: Insulin receptor substrate 1; LLC: lung carcinoma; LPC: lysophosphatidylcholine; MAVS: mitochondrial antiviral signaling; MLK3: mixed lineage kinase 3; MLKL: mixed lineage kinase domain-like; MMP: matrix metalloproteinasse; MVs: microvesicles; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; PA: Palmitate; PAI-1: plasminogen activator inhibitor; PAR: protease-activated receptor; PBMC: peripheral blood mononuclear cell; PC: pancreatic cancer; PDAC: pancreatic ductal adenocarcinoma; PMFs: portal myofibroblasts; PPARγ: peroxisome proliferator-activated receptor γ; PS: phosphatidylserine; RIPK3: receptor-interacting protein kinase-3; ROCK1: rho-associated, coiled-coil-containing protein kinase 1; SAT: subcutaneous adipose tissue; S1P: sphingosine-1-phosphate; STAT3: transcription 3; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; TF: tissue factor; TIMP: tissue inhibitors of metalloproteinase; TLR4: Toll-like receptor-4; TNF-α: tumor necrosis factor-α; TRIF: Toll-interleukin-1 receptor domain-containing adaptor protein inducing interferon-β; tsRNAs: tRNA-derived small RNAs; TSE: tobacco smoke extracts; TGF-β: transforming growth factor beta; TRAIL: tumor necrosis factor-like apoptosis inducing ligand; TRAIL-R2: tumor necrosis factor-like apoptosis inducing ligand receptor 2; UCP1: uncoupling protein 1; VAT: visceral adipose tissue; VEGF-A: vascular endothelial growth factor A; WAT: white adipose tissue.
Acknowledgements
This work was partially supported by Grants from Natural Science Foundation of Tianjin (19JCZDJC36100 to C. J. L and 18ZXDBSY22120 to J. N. L), and Lupus Research Alliance (416805, M. L. L) and NIH R21AI144838 (to M. L. L).
Author Contributions
Q. H. F and C. J. L wrote the main text. J. N. L and M. L. L conceptualized the study. C. J. L and M. L. L designed the figures. M. L. L and J. N. L contributed in researching the content for the review, discussion of the content and editing before submission.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Matta J, Carette C, Rives Lange C, Czernichow S. French and worldwide epidemiology of obesity. Presse Med. 2018;47:434-438
2. Seganfredo FB, Blume CA, Moehlecke M, Giongo A, Casagrande DS, Spolidoro JVN. et al. Weight-loss interventions and gut microbiota changes in overweight and obese patients: a systematic review. Obes Rev. 2017;18:832-851
3. Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA. et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17:212
4. Farpour-Lambert NJ, Baker JL, Hassapidou M, Holm JC, Nowicka P, O'Malley G. et al. Childhood Obesity Is a Chronic Disease Demanding Specific Health Care-a Position Statement from the Childhood Obesity Task Force (COTF) of the European Association for the Study of Obesity (EASO). Obes Facts. 2015;8:342-9
5. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1-4
6. Rendón-Macías ME, Rosas-Vargas H, Villasís-Keever MÁ, Pérez-García C. Children's perception on obesity and quality of life: a Mexican survey. BMC Pediatr. 2014;14:131
7. Li M, Li C, Liu Y, Chen Y, Wu X, Yu D. et al. Decreased secretion of adiponectin through its intracellular accumulation in adipose tissue during tobacco smoke exposure. Nutr Metab (Lond). 2015;12:15
8. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E. et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250-2
9. Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR. et al. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402-5
10. Liu ML, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19:121-7
11. Wu X, Liu Y, Wei W, Liu ML. Extracellular vesicles in autoimmune vasculitis - Little dirts light the fire in blood vessels. Autoimmun Rev. 2019;18:593-606
12. Liu ML, Williams KJ, Werth VP. Microvesicles in Autoimmune Diseases. Adv Clin Chem. 2016;77:125-175
13. Lim CZJ, Zhang L, Zhang Y, Sundah NR, Shao H. New sensors for extracellular vesicles: insights on constituent and associated biomarkers. ACS Sens. 2019;5:4-12
14. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB. et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509-17
15. Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R. et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864-71
16. Liu ML, Werth VP, Williams KJ. Blood plasma versus serum: which is right for sampling circulating membrane microvesicles in human subjects? Ann Rheum Dis. 2019
17. Stepanian A, Bourguignat L, Hennou S, Coupaye M, Hajage D, Salomon L. et al. Microparticle increase in severe obesity: not related to metabolic syndrome and unchanged after massive weight loss. Obesity (Silver Spring). 2013;21:2236-43
18. Eguchi A, Lazic M, Armando AM, Phillips SA, Katebian R, Maraka S. et al. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl). 2016;94:1241-1253
19. Chen Y, Li G, Liu ML. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genomics Proteomics Bioinformatics. 2018;16:50-62
20. Latifkar A, Hur YH, Sanchez JC, Cerione RA, Antonyak MA. New insights into extracellular vesicle biogenesis and function. J Cell Sci. 2019 132
21. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329-39
22. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-78
23. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the tsferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942-8
24. Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab. 2017;28:3-18
25. van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P. et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708-21
26. Liu ML, Scalia R, Mehta JL, Williams KJ. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arterioscler Thromb Vasc Biol. 2012;32:2113-21
27. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269-88
28. Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31:15-26
29. Li CJ, Liu Y, Chen Y, Yu D, Williams KJ, Liu ML. Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am J Pathol. 2013;182:1552-62
30. Folkesson M, Li C, Frebelius S, Swedenborg J, Wågsäter D, Williams KJ. et al. Proteolytically active ADAM10 and ADAM17 carried on membrane microvesicles in human abdominal aortic aneurysms. Thromb Haemost. 2015;114:1165-74
31. Schreiber A, Rousselle A, Becker JU, von Massenhausen A, Linkermann AA-O, Kettritz RA-O. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A. 2017;114:E9618-E9625
32. Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity. 2017;47:51-65
33. Kolb JP, Oguin TH, Oberst A, Martinez J. Programmed Cell Death and Inflammation: Winter Is Coming. Trends Immunol. 2017;38:705-718
34. Zargarian S, Shlomovitz I, Erlich Z, Hourizadeh A, Ofir-Birin Y, Croker BA. et al. Phosphatidylserine externalization, "necroptotic bodies" release, and phagocytosis during necroptosis. PLoS Biol. 2017;15:e2002711
35. Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590-604
36. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-5
37. Luan J, Chen W, Fan J, Wang S, Zhang X, Zai W. et al. GSDMD membrane pore is critical for IL-1β release and antagonizing IL-1β by hepatocyte-specific nanobiologics is a promising therapeutics for murine alcoholic steatohepatitis. Biomaterials. 2020 119570
38. Van Opdenbosch N, Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50:1352-1364
39. Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr Rev. 2019;40:1367-1393
40. Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509-520
41. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99-109
42. Wree A, Holtmann TM, Inzaugarat ME, Feldstein AE. Novel Drivers of the Inflammatory Response in Liver Injury and Fibrosis. Semin Liver Dis. 2019;39:275-282
43. Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci Rep. 2018;8:8973
44. Starr ME, Saito M, Evers BM, Saito H. Age-Associated Increase in Cytokine Production During Systemic Inflammation-II: The Role of IL-1beta in Age-Dependent IL-6 Upregulation in Adipose Tissue. J Gerontol A Biol Sci Med Sci. 2015;70:1508-15
45. Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA. et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997
46. Takasugi M, Okada R, Takahashi A, Virya Chen DA-O, Watanabe SA-O, Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun. 2017;8:15729
47. Desdin-Mico G, Mittelbrunn M. Role of exosomes in the protection of cellular homeostasis. Cell Adh Migr. 2017;11:127-134
48. Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N. et al. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019;30:329-342
49. Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509-20
50. Fábrega MJ, Aguilera L, Giménez R, Varela E, Alexandra Cañas M, Antolín M, Activation of Immune, Defense Responses in the Intestinal Mucosa by Outer Membrane Vesicles of Commensal, Probiotic Escherichia coli Strains. Front Microbiol. 2016; 7: 705.
51. Hubal MJ, Nadler EP, Ferrante SC, Barberio MD, Suh JH, Wang J. et al. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25:102-110
52. Jayabalan N, Lai A, Ormazabal V, Adam S, Guanzon D, Palma C. et al. Adipose Tissue Exosomal Proteomic Profile Reveals a Role on Placenta Glucose Metabolism in Gestational Diabetes Mellitus. J Clin Endocrinol Metab. 2019;104:1735-1752
53. Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M. et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327-33
54. Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y. et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498-505
55. Koeck ES, Iordanskaia T, Sevilla S, Ferrante SC, Hubal MJ, Freishtat RJ. et al. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res. 2014;192:268-75
56. Rouch A, Vanucci-Bacque C, Bedos-Belval F, Baltas M. Small molecules inhibitors of plasminogen activator inhibitor-1 - an overview. Eur J Med Chem. 2015;92:619-36
57. Ramachandran P, Iredale JP. Liver fibrosis: a bidirectional model of fibrogenesis and resolution. QJM. 2012;105:813-7
58. Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59:1075-88
59. Loncar D. Convertible adipose tissue in mice. Cell Tissue Res. 1991;266:149-61
60. Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Occurrence of brown adipocytes in rat white adipose tissue. molecular and morphological characterization. J Cell Sci. 1992;103:931-42
61. Jung YJ, Kim HK, Cho Y, Choi JS, Woo CH, Lee KS. et al. Cell reprogramming using extracellular vesicles from differentiating stem cells into white/beige adipocytes. Sci Adv. 2020;6:eaay6721
62. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450-455
63. Chen Y, Buyel JJ, Hanssen MJ, Siegel F, Pan R, Naumann J. et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun. 2016;7:11420
64. Chen Y, Siegel F, Kipschull S, Haas B, Fröhlich H, Meister G. et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun. 2013;4:1769
65. Heinrich LF, Andersen DK, Cleasby ME, Lawson C. Long-term high fat feeding of rats results in increased numbers of circulating microvesicles with pro-inflammatory effects on endothelial cells. Br J Nutr. 2015;113(11):1704-11
66. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7(1):81-96
67. Stępień EŁ, Durak-Kozica M, Kamińska A, Targosz-Korecka M, Libera M, Tylko G. et al. Circulating ectosomes: Determination of angiogenic microRNAs in type 2 diabetes. Theranostics. 2018;8:3874-3890
68. Royo F, Palomo L, Mleczko J, Gonzalez E, Alonso C, Martínez I. et al. Metabolically active extracellular vesicles released from hepatocytes under drug-induced liver-damaging conditions modify serum metabolome and might affect different pathophysiological processes. Eur J Pharm Sci. 2017;98:51-57
69. Royo F, Moreno L, Mleczko J, Palomo L, Gonzalez E, Cabrera D. et al. Hepatocyte-secreted extracellular vesicles modify blood metabolome and endothelial function by an arginase-dependent mechanism. Sci Rep. 2017;7:42798
70. Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577-91
71. Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA. et al. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell. 2018;175:695-708
72. Chen Y, Li G, Liu Y, Werth VP, Williams KJ, Liu ML. Translocation of Endogenous Danger Signal HMGB1 From Nucleus to Membrane Microvesicles in Macrophages. J Cell Physiol. 2016;231:2319-26
73. Chen CM, Chou HC. Human mesenchymal stem cells attenuate hyperoxia-induced lung injury through inhibition of the renin-angiotensin system in newborn rats. Am J Transl Res. 2018;10:2628-2635
74. Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB. et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017;171:372-384
75. Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H. et al. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes. 2018;67:235-247
76. Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL. et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456-64
77. Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 2011;89:417-21
78. Nathanielsz PW, Yan J, Green R, Nijland M, Miller JW, Wu G. et al. Maternal obesity disrupts the methionine cycle in baboon pregnancy. Physiol Rep. 2015 3
79. Desai M, Han G, Ross MG. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite. 2016;99:193-9
80. Su L, Patti ME. Paternal Nongenetic Intergenerational Transmission of Metabolic Disease Risk. Curr Diab Rep. 2019;19:38
81. Isganaitis E, Suehiro H, Cardona C. Who's your daddy?: paternal inheritance of metabolic disease risk. Curr Opin Endocrinol Diabetes Obes. 2017;24:47-55
82. Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397-400
83. Sarker G, Sun W, Rosenkranz D, Pelczar P, Opitz L, Efthymiou V. et al. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc Natl Acad Sci U S A. 2019;116:10547-10556
84. Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY. et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391-396
85. Sales VM, Ferguson-Smith AC, Patti ME. pigenetic Mechanisms of Transmission of Metabolic Disease Across Generations. Cell Metab. 2017;25:559-571
86. Jaeger K, Saben JL, Moley KH. Transmission of Metabolic Dysfunction Across Generations. Physiology (Bethesda). 2017;32:51-59
87. Maciel E, Mansuy IM. Extracellular Vesicles and their miRNA Cargo: A Means of Communication between Soma and Germline in the Mammalian Reproductive System. Chimia (Aarau). 2019;73:356-361
88. Morgan CP, Chan JC, Bale TL. Driving the Next Generation: Paternal Lifetime Experiences Transmitted via Extracellular Vesiclesand Their Small RNA Cargo. Biol Psychiatry. 2019;85:164-171
89. Chan JC, Morgan CP, Adrian Leu N, Shetty A, Cisse YM, Nugent BM. et al. Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat Commun. 2020;11:1499
90. Sharma U. Paternal Contributions to Offspring Health: Role of Sperm Small RNAs in Intergenerational Transmission of Epigenetic Information. Front Cell Dev Biol. 2019;7:215
91. Conine CC, Sun F, Song L, Rivera-Pérez JA, Rando OJ. Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev Cell. 2019;46:470-480
92. Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update. 2016;22:182-193
93. Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;7:432-9
94. Wu L, Lu Y, Jiao Y, Liu B, Li S, Li Y. et al. Paternal psychological stress reprograms hepatic gluconeogenesis in offspring. Cell Metab. 2016 23, 735-743
95. Siklenka K, Erkek S, Godmann M, Lambrot R, McGraw S, Lafleur C. et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015 350, 6261
96. Wang Y, Chen LM, Liu ML. Microvesicles and diabetic complications-novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacol Sin. 2014;35:433-43
97. Kobayashi Y, Eguchi A, Tempaku M, Honda T, Togashi K, Iwasa M. et al. Circulating extracellular vesicles are associated with lipid and insulin metabolism. Am J Physiol Endocrinol Metab. 2018;315:E574-E582
98. Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG. et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840-5
99. Leroyer AS, Tedgui A, Boulanger CM. Microparticles and type 2 diabetes. Diabetes Metab. 2008;34(Suppl 1):S27-32
100. Ban LA, Shackel NA, McLennan SV. Extracellular Vesicles: A New Frontier in Biomarker Discovery for Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17:376
101. Ge Q, Xie XX, Xiao X, Li X. Exosome-Like Vesicles as New Mediators and Therapeutic Targets for Treating Insulin Resistance and beta-Cell Mass Failure in Type 2 Diabetes Mellitus. J Diabetes Res. 2019;2019:3256060
102. Jalabert A, Vial G, Guay C, Wiklander OP, Nordin JZ, Aswad H. et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016;59:1049-58
103. Aswad H, Forterre A, Wiklander OP, Vial G, Danty-Berger E, Jalabert A. et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014;57:2155-2164
104. Yu Y, Du H, Wei S, Feng L, Li J, Yao F. et al. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle through Repression of PPARgamma. Theranostics. 2018;8:2171-2188
105. Kranendonk ME, Visseren FL, van Herwaarden JA, Nolte-'t Hoen EN, de Jager W, Wauben MH. et al. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring). 2014;22:2216-23
106. Mleczko J, Ortega FJ, Falcon-Perez JM, Wabitsch M, Fernandez-Real JM, Mora S. Extracellular Vesicles from Hypoxic Adipocytes and Obese Subjects Reduce Insulin-Stimulated Glucose Uptake. Mol Nutr Food Res. 2018;62:1700917
107. Garneau L, Aguer C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. 2019; 45: 505-516.
108. Wieser V, Adolph TE, Grander C, Grabherr F, Enrich B, Moser P. et al. Adipose type I interferon signalling protects against metabolic dysfunction. Gut. 2018;67:157-165
109. Li M, Yu D, Williams KJ, Liu ML. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler Thromb Vasc Biol. 2010;30:1818-24
110. Song M, Han L, Chen FF, Wang D, Wang F, Zhang L. et al. Adipocyte-Derived Exosomes Carrying Sonic Hedgehog Mediate M1 Macrophage Polarization-Induced Insulin Resistance via Ptch and PI3K Pathways. Cell Physiol Biochem. 2018;48:1416-1432
111. Hart KM, Fabre T, Sciurba JC, Gieseck RL 3rd, Borthwick LA, Vannella KM. et al. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-β. Sci Transl Med. 2017;9:eaal3694
112. Li Y, Zeidi M, Bashir M, Werth VP, Liu M. Extracellular MAVS associates with microvesicles that can actively trigger IFNβ production. J Invest Dermatol. 2019;139:S9
113. Garcia-Contreras M, Brooks RW, Boccuzzi L, Robbins PD, Ricordi C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017;21:2940-2956
114. Dotta F, Ventriglia G, Snowhite IV, Pugliese A. MicroRNAs: markers of beta-cell stress and autoimmunity. Curr Opin Endocrinol Diabetes Obes. 2018;25:237-245
115. Association AD. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S13-S28
116. Rutman AK, Negi S, Gasparrini M, Hasilo CP, Tchervenkov J, Paraskevas S. Immune Response to Extracellular Vesicles From Human Islets of Langerhans in Patients With Type 1 Diabetes. Endocrinology. 2018;159:3834-3847
117. Guay C, Kruit JK, Rome S, Menoud V, Mulder NL, Jurdzinski A. et al. Lymphocyte-Derived Exosomal MicroRNAs Promote Pancreatic β Cell Death and May Contribute to Type 1 Diabetes Development. Cell Metab. 2019;29:348-361
118. Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe Ahmed M. et al. Primary Human and Rat beta-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes. 2017;66:460-473
119. Lakhter AJ, Pratt RE, Moore RE, Doucette KK, Maier BF, DiMeglio LA. et al. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia. 2018;61:1124-1134
120. Barutta F, Tricarico M, Corbelli A, Annaratone L, Pinach S, Grimaldi S. et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One. 2013;8:e73798
121. Li S, Wei J, Zhang C, Li X, Meng W, Mo X. et al. Cell-Derived Microparticles in Patients with Type 2 Diabetes Mellitus: a Systematic Review and Meta-Analysis. Cell Physiol Biochem. 2016;39:2439-2450
122. Fu Q, Li Y, Jiang H, Shen Z, Gao R, He Y. et al. Hepatocytes derived extracellular vesicles from high-fat diet induced obese mice modulate genes expression and proliferation of islet β cells. Biochem Biophys Res Commun. 2019;516:1159-1166
123. Chen Y, Li G, Liu Y, Williams KJ, Liu ML. Exposure of human macrophages to tobacco smoke induces Hmgb1 release on microvesicles that cause monocyte recruitment and impairment of insulin signaling in adipocytes. Arterioscler Thromb Vasc Biol. 2013;33:A162
124. Ali S, Vergori L, Soleti R, Le Lay S, Simard G, Dubois S. et al. Circulating exosomes from metabolic syndrome patients induce insulin resistance in human hepatocytes but not in human endothelial cells. Archives of Cardiovascular Diseases Supplements. 2019;11:188
125. Lichtenauer M, Jung C. Microvesicles and ectosomes in angiogenesis and diabetes - message in a bottle in the vascular ocean. Theranostics. 2018;8:3974-3976
126. Osmai M, Osmai Y, Bang-Berthelsen CH, Pallesen EMH, Vestergaard AL, Novotny GW. et al. MicroRNAs as regulators of beta-cell function and dysfunction. Diabetes Metab Res Rev. 2016;32:334-49
127. Rossi L, Nicoletti MC, Carmosino M, Mastrofrancesco L, Di Franco A, Indrio F. et al. Urinary Excretion of Kidney Aquaporins as Possible Diagnostic Biomarker of Diabetic Nephropathy. J Diabetes Res. 2017;2017:4360357
128. Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U. et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. FASEB J. 2015;29:589-96
129. Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627-36
130. Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445-51
131. Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A. et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88
132. Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW. et al. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956-67
133. Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF. et al. Petra Hirsova, Samar H. Ibrahim, Vikas K. Verma, et al. Extracellular Vesicles in Liver Pathobiology: Small Particles with Big Impact. Hepatology. 2016;64:2219-2233
134. Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455-466
135. Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA. et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731-44
136. Ibrahim SH, Gores GJ, Hirsova P, Kirby M, Miles L, Jaeschke A. et al. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver international: official journal of the International Association for the Study of the Liver. 2014;34:427-437
137. Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57:233-45
138. Bruno S, Pasquino C, Herrera Sanchez MB, Tapparo M, Figliolini F, Grange C. et al. HLSC-Derived Extracellular Vesicles Attenuate Liver Fibrosis and Inflammation in a Murine Model of Non-alcoholic Steatohepatitis. Mol Ther. 2020;28:479-489
139. Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S. et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859-64
140. Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L. et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell Mol Gastroenterol Hepatol. 2015;1:646-663
Author contact
![]() Corresponding authors: E-mails: linjingnanet.cn (Jing-Na Lin); li_chunjuncom (Chun-Jun Li); lium1upenn.edu (Ming-Lin Liu).
Corresponding authors: E-mails: linjingnanet.cn (Jing-Na Lin); li_chunjuncom (Chun-Jun Li); lium1upenn.edu (Ming-Lin Liu).
 Global reach, higher impact
Global reach, higher impact