13.3
Impact Factor
Theranostics 2020; 10(17):7561-7580. doi:10.7150/thno.41802 This issue Cite
Research Paper
Exosomes-mediated Transfer of miR-125a/b in Cell-to-cell Communication: A Novel Mechanism of Genetic Exchange in the Intestinal Microenvironment
1. Research Institute of General Surgery, Jinling Hospital, Medical School of Nanjing University, Nanjing, 210000, China.
2. State Key Laboratory of Pharmaceutical Biotechnology, Jiangsu Engineering Research Center for MicroRNA Biology and Biotechnology, School of Life Sciences, NJU Advanced Institute for Life Sciences, Nanjing University, Nanjing, 210000, China.
3. Department of General Surgery, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, 210000, China.
4. Department of General Surgery, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221000, People's Republic of China.
5. Department of General Surgery, Jiangyin Hospital Affiliated to Nantong University, Jiangyin, Jiangsu, 214400, People's Republic of China.
*These authors contributed equally to this work.
Abstract
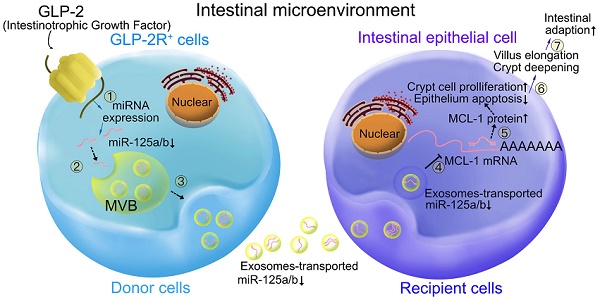
Glucagon-like peptide-2 (GLP-2), a key factor in intestinal rehabilitation therapy of short bowel syndrome (SBS), may require cell-to-cell communication to exert its biological functions. However, understanding of the mechanism remains elusive. Here, we report participation of exosomal miR-125a/b in GLP-2 mediated intestinal epithelial cells-myofibroblasts cross-talk in intestinal microenvironment.
Methods: The effects of GLP-2 on the proliferation and apoptosis of intestinal epithelial cells in SBS rat models were evaluated. Exosomes were extracted from residual jejunum tissue of GLP-2 or vehicle treated SBS rats using ultracentrifugation method, and identified by nanoparticle trafficking analysis (NTA), transmission electron microscopy and western blotting. miRNA sequencing combined with qRT-PCR validation were used to identify differentially expressed miRNAs. miRNAs, which might be involved in proliferation and apoptosis of intestinal epithelial cells, were screened and further verified by miRNA functional experiments. Moreover, the proliferation-promoting and anti-apoptosis effects of GLP-2 on intestinal myofibroblasts, which expressing GLP-2 receptor, and whether GLP-2 could influence the content of miRNAs in the derived exosomes were studied. The downstream pathways were explored by miRNA function recovery experiment, luciferase reporter assay, pull down experiment, knockdown and overexpression of target gene and other experiments based on the bioinformatics prediction of miRNA target gene.
Results: GLP-2 significantly promoted intestinal growth, facilitated the proliferation of intestinal crypt epithelial cells and inhibited the apoptosis of intestinal villi epithelial cells in type II SBS rats. GLP-2 significantly down-regulated exosomal miR-125a/b both in residual jejunums derived exosomes and in exosomes secreted by GLP-2R positive cells. Exosomal miR-125a/b was responsible for GLP-2 mediated intestinal epithelial cells proliferation promotion and apoptosis attenuation. miR-125a/b inhibited the proliferation and promotes apoptosis of intestinal epithelial cells by suppressing the myeloid cell leukemia-1 (MCL1).
Conclusions: miR-125a/b shuttled by intestinal myofibroblasts derived exosomes regulate the proliferation and apoptosis of intestinal epithelial cells. GLP-2 treatment significantly decreases the level of miR-125a/b in the exosomes of intestinal myofibroblasts. miR-125a/b modulates the proliferation and apoptosis of intestinal epithelial cells by targeting the 3'UTR region of MCL1. Hence, this study indicates a novel mechanism of genetic exchange between cells in intestinal microenvironment.
Keywords: Exosomes, Glucagon-like peptide-2, miR-125a/b, Short bowel syndrome, Proliferation and apoptosis of intestinal epithelial cells
 Global reach, higher impact
Global reach, higher impact