13.3
Impact Factor
Theranostics 2020; 10(17):7747-7757. doi:10.7150/thno.46082 This issue Cite
Research Paper
Acetylation dependent functions of Rab22a-NeoF1 Fusion Protein in Osteosarcoma
1. Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China.
2. Fujian Provincial Key Laboratory of Innovative Drug Target Research, School of Pharmaceutical Sciences, Xiamen University, Xiang'an South Road, Xiamen, Fujian 361102, China; State Key Laboratory of Cellular Stress Biology, Xiamen University, Xiang'an South Road, Xiamen, Fujian 361102, China.
Abstract
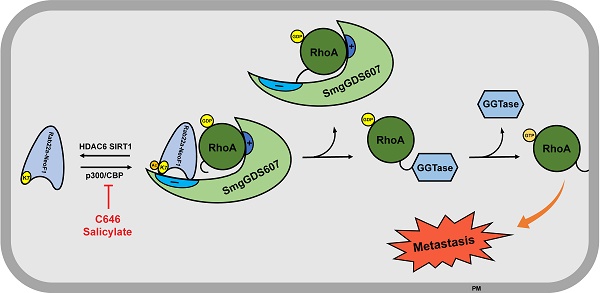
Background: Rab22a-NeoF1 fusion gene containing the 1-38aa of Rab22a (Rab22a1-38) plays a decisive role in driving tumor metastasis by activating RhoA via binding to SmgGDS607. However, its intercellular regulation remains unknown.
Methods: The Lys7 (K7) acetylation of Rab22a-NeoF1 was initially identified by mass spectrum. Co-transfection, immunoprecipitation and Western blotting were used to characterize the acetyltransferases and deacetylases responsible for the K7 acetylation of Rab22a-NeoF1, and to define the interaction of proteins. The specificity of K7 acetylation of Rab22a-NeoF1 was determined by its specific anti-K7ac-Rab22a-NeoF1 antibody and its K7R mutant. RhoA-GTP was measured by RhoA activation assay. The migration and invasion were assessed by Transwell assay without and with Matrigel matrix, respectively. The orthotopic osteosarcoma metastasis model in vivo was used to monitor the lung metastases of U2OS/MTX300-Luc stably expressing Vector, Rab22a-NeoF1 or its K7R mutant with or without C646, a relatively specific inhibitor of p300/CBP. The unpaired Student t test was used for the statistical significance.
Results: The K7 of Rab22a-NeoF1 is acetylated by p300/CBP while is de-acetylated by both HDAC6 and SIRT1. The K7R mutant of Rab22a-NeoF1 lacks its binding to SmgGDS607 and subsequently lost its promoting functions, such as activation of RhoA, cell migration, invasion and lung metastasis in osteosarcoma in vitro and in vivo, which are also diminished by p300/CBP inhibitor C646.
Conclusion:The promoting function of Rab22a-NeoF1 is dependent on its K7 acetylation in osteosarcoma, and targeting this acetylation (e.g., C646) may benefit cancer patients, in particular osteosarcoma patients, who are positive for the Rab22a1-38.
Keywords: osteosarcoma, Rab22a-NeoF1, acetylation, metastasis, p300/CBP
 Global reach, higher impact
Global reach, higher impact