13.3
Impact Factor
Theranostics 2020; 10(17):7812-7820. doi:10.7150/thno.47251 This issue Cite
Research Paper
Analysis of PSMA expression and outcome in patients with advanced Prostate Cancer receiving 177Lu-PSMA-617 Radioligand Therapy
1. Department of Nuclear Medicine, University Hospital Münster, Münster, Germany.
2. Department of Nuclear Medicine, University Hospital Essen, Essen, Germany.
3. German Cancer Consortium (DKTK).
4. West German Cancer Center.
5. Department of Urology, University Hospital Münster, Münster, Germany.
6. Division of Radiology, German Cancer Research Center, Heidelberg, Germany.
Received 2020-4-21; Accepted 2020-5-22; Published 2020-6-19
Abstract
Rationale: PSMA-PET-CT enables measuring molecular expression of prostate-specific membrane antigen (PSMA) in vivo, which is the target molecule of 177Lu-PSMA-617 (Lu-PSMA) therapy. However, the correlation of PSMA expression and overall survival (OS) in patients treated with Lu-PSMA therapy is currently unclear; especially with regard to coexistence of high and low PSMA expressing metastases. To this end, this retrospective single arm study elucidates the correlation of PSMA expression and overall survival in patients treated with Lu-PSMA therapy. Additionally, PET based criteria to define low PSMA expression were explored.
Methods: Eighty-five patients referred to Lu-PSMA therapy were included in the analysis. Pretherapeutic 68Ga-PSMA-PET-CT scans were available for all patients. SUVmax of the highest PSMA expressing metastasis (PSMAmax), SUVmax of the lowest PSMA expressing metastasis (PSMAmin), and average SUVmax of all metastases (PSMAaverage) amongst other PET parameters were measured for each patient. A log-rank cutoff-finder was used to determine low (lowPSMAaverage) and high (highPSMAaverage) average PSMA expression as well as low (lowPSMAmin) and high (highPSMAmin) minimal PSMA expression.
Results: PSMAaverage was a significant prognosticator of overall survival in contrast to PSMAmax (HR: 0.959; p = 0.047 vs. HR: 0.992; p = 0.231). Optimal log rank cut-offs were: PSMAaverage = 14.3; PSMAmin = 10.2. Patients with low average PSMA expression (lowPSMAaverage) had significantly shorter survival compared to those with high average expression (highPSMAaverage) (5.3 vs. 15.1 months; p < 0.001; HR: 3.738, 95%CI = 1.953-7.154; p < 0.001). Patients with low PSMA expressing metastases (lowPSMAmin) had shorter survival compared to those without a low PSMA expressing metastasis (highPSMAmin) (p = 0.003; 7.9 months vs. 21.3; HR: 4.303, 95%CI = 1.521-12.178; p = 0.006). Patients that were classified as highPSMAaverage but with lowPSMAmin had an intermediate overall survival (11.4 months; longer compared to lowPSMAaverage, 5.3 months, p = 0.002; but shorter compared to highPSMAmin, 21.3 months, p = 0.02).
Conclusion: Low average PSMA expression is a negative prognosticator of overall survival. Absence of low PSMA expressing metastases is associated with best overall survival and the maximum PSMA expression seems not suited to prognosticate overall survival. Low PSMA expression might therefore be a negative prognosticator for the outcome of patients treated with Lu-PSMA therapy. Future studies are warranted to elucidate the degree of low PSMA expression tolerable for Lu-PSMA therapy.
Keywords: PSMA radioligand therapy, PSMA PET, prostate cancer, prognosticator
Introduction
There are only limited therapeutic options for patients with metastatic castration-resistant prostate cancer (mCRPC) [1]. However, the treatment of mCRPC patients with 177Lu-PSMA-617 (Lu-PSMA) achieves biochemical response (> 50% decline of prostate-specific antigen blood levels) in 45-64% of patients [2-4]. Yet, the identification of mCRPC patients who will benefit from Lu-PSMA therapy is still an unmet clinical issue [5,6].
Prostate-specific membrane antigen (PSMA) targeted positron emission tomography computed tomography (PET-CT) can visualize the target molecule of Lu-PSMA therapy in vivo, which is also referred to as theranostics [6-9]. The molecular expression of PSMA should be directly linked to Lu-PSMA efficacy. Therefore, procedure guidelines of the European Association of Nuclear Medicine (EANM) for Lu-PSMA therapy demand a PSMA-PET-CT acquisition to evaluate therapy eligibility [10]. However, there are contradictory reports on the implications of PSMA targeted imaging: It has been reported that PSMA-PET is not suited to predict response to Lu-PSMA therapy [11]. On the other hand, high tumor uptake in post Lu-PSMA therapy scintigraphies is a prognosticator of survival [12]. It remains currently unclear to what extent PSMA expression measured by PSMA-PET-CT can predict response and prognosticate overall survival and thus, ultimately assess eligibility for Lu-PSMA therapy. Moreover, there is no reasonable definition of low PSMA expression.
The first prospective Phase II trial by Hofman et al. has addressed the issue of eligibility assessment pragmatically by requesting an arbitrarily defined minimum PSMA-PET uptake of metastases to undergo Lu-PSMA therapy [3,13]. The minimum PSMA uptake of any metastasis was defined as 1.5 times the mean liver uptake [3]. Only patients whose SUVmax exceeded this minimum activity at any metastatic site were eligible for Lu-PSMA therapy. It seems plausible that PSMA-PET uptake should be linked to therapeutic efficacy of Lu-PSMA therapy. However, it appears difficult to translate the trial inclusion criteria to the clinical routine, as it was not part of the study evaluation. By applying a SUVmax based criterion, Lu-PSMA therapy might be withheld from patients due to their low PSMA expression that still might have benefited from therapy. Therefore, the aim of the present study was to investigate the relevance of PSMA-PET parameters for the overall survival of patients treated by Lu-PSMA therapy. Additionally, multiple PSMA-PET parameters were employed to distinguish between patients with low and high PSMA expression. Finally, survival time, presence of liver metastases and history of second line chemotherapy were compared between patients with low and high PSMA expression.
Methods
Patients
All patients who were referred for 177Lu-PSMA-617 therapy at the Department of Nuclear Medicine in Muenster between December 2014 and October 2018 were considered in this retrospective analysis. Inclusion criterion was the presence of a 68Ga-PSMA-11 PSMA-PET-CT examination prior to administration of the first therapy cycle showing any uptake of the tracer in the target lesions. The decision for Lu-PSMA-617 therapy was made by the institutional interdisciplinary tumor board on a case by case basis. Prerequisites for 177Lu-PSMA-617 therapy were: castration-resistance (mCRPC), sustained androgen deprivation therapy, if no contraindication was present at least one line of taxane chemotherapy, PSMA-positive metastases, sufficient hematological reserve, and sufficient kidney as well as liver function [10]. Pretherapeutic PSMA-PETs were assessed visually for presence of PSMA positive metastases; no quantitative PSMA-PET related inclusion criteria were applied. Foci that were not caused by physiological uptake and showed higher activity than the surrounding tissue were assessed as sufficient for Lu-PSMA therapy. A detailed patient characteristic is given by Table 1.
PSMA-PET imaging procedure
68Ga-PSMA-11 was produced according to manufactures recommendations (precursor delivered by ABX GmbH, Radeberg, Germany). A Siemens Biograph mCT (Siemens Healthineers, Knoxville, TN, United States) was used for image acquisition. PET-CT acquisitions were started 60 minutes after tracer injection. Low-dose or diagnostic CT were acquired immediately prior to PET acquisition for anatomical orientation and attenuation correction. PET reconstruction was done using the standard software as provided by the manufacturer and an iterative time-of-flight algorithm without PSF correction. The median interval between PET acquisition and therapy start was 32 (IQR: 22) days.
PSMA therapy preparation and administration
177Lu-PSMA-617 was prepared as described elsewhere (Lutetium: ITG Isotopes Technology, Garching, Germany; precursor: ABX advanced biochemical compounds, Radeberg, Germany) [14]. Lu-PSMA was administered every 8 weeks until severe adverse reactions, altered therapy regime, progression, or death occurred.
Patient characteristics
| Patient characteristics | N [%] | Median [IQR]; survival: Median [CI] |
|---|---|---|
| Number of patients | 85 [100%] | |
| Age (years) | 73.1 [11.4] | |
| Estimated overall survival time (months) | 11.4 [8.0-14.7] | |
| >50% PSA decline from baseline | 39 [46%]; n = 80, follow up not present for 5 patients. | |
| PSMA therapy | ||
| Number of cycles | 3.0 [4] | |
| Cumulated activity (GBq) | 19.3 [24.8] | |
| Baseline blood parameters | ||
| Alkaline phosphatase (U/l) | 147.0 [193.0] | |
| Lactate dehydrogenase (U/l) | 316.5 [227.0] | |
| Aspartate aminotransferase (U/l) | 32.5 [24.0] | |
| Alanine transaminase (U/l) | 16.0 [11.0] | |
| Hemoglobin (g/dl) | 10.4 [2.4] | |
| Prostate-specific antigen (ng/ml) | 284.0 [805.0] | |
| Metastases | ||
| Bone | 78 [92%] | |
| Lymph node | 68 [80%] | |
| Liver | 26 [31%] | |
| Lung | 20 [24%] | |
| Brain | 1 [1%] | |
| Previous therapies | ||
| Docetaxel | 68 [80%] | |
| Cabazitaxel | 20 [24%] | |
| Abiraterone | 72 [85%] | |
| Enzalutamide | 72 [85%] | |
Blood parameters were not available for all patients; Abbreviations: Std = standard deviation; CI = confidence interval.
PSMA-PET image analysis
The analysis of PSMA-PET images was done semi-automatically using the research prototype software MI Whole Body Analysis Suite (MIWBAS, v1.0, Siemens Medical Solutions USA, Inc., Knoxville, TN), which has been described in detail before [15]. Briefly, all PSMA avid foci were automatically pre-selected based by a pre-defined threshold; foci with a PET volume smaller than 0.5 ml (segmented by 50% of local SUVmax as threshold) were discarded. Missed pathological foci were manually added, if necessary. PSMA avid foci that were caused by physiological tracer accumulation were semi automatically removed from the analysis.
All PSMA avid metastases (regardless of SUVmax) were segmented and SUVmax, SUVmean, SUVpeak were reported for each metastasis. Metastases were delineated using relative thresholding (50% of local SUVmax). On a per patient level, the mean of all SUVmax measurements (PSMAaverage), the maximum SUVmax measurement (PSMAmax), the lowest SUVmax measurement (PSMAmin) and the standard deviation of the SUVmax measurements (PSMAstd) were noted.
PSMA measurements were analyzed as continuous parameter and in binarized form. When PSMAaverage was binarized using optimized log rank thresholds, the group with low PSMAaverage was denoted lowPSMAaverage (highPSMAaverage; threshold: 14.3). When PSMAmin was binarized, patients with low PSMA expressing metastases were denoted lowPSMAmin (without low PSMA expressing metastases: highPSMAmin; threshold: 10.2). The volumetric fraction of low PSMA expressing tumor was determined by dividing the volume of metastases with low PSMA expression (SUVmax ≤ 10.2) by the whole-body tumor volume.
Statistical analysis
SPSS 25 (IBM, NY, USA) was used for log rank tests, Pearson correlation, Mann-Whitney-U test, Fisher's exact test and uni- as well as multivariate Cox-regression. R and the maxstat package were used for finding the optimal log rank cut-off of continuous variables [16,17]. P values < 0.05 were regarded as statistically significant. To correct for log-rank test alpha error accumulation, significance was assumed when p < 0.0125 (Bonferroni correction for 4 SUVmax tests: optimal log rank cut-off for PSMAmax, PSMAmin, PSMAaverage, PSMAstd. Other SUV parameters (SUVmean, SUVpeak) were only analyzed to further corroborate SUVmax findings and therefore not regarded for Bonferroni correction. Values are presented together with the interquartile range (IQR).
Results
PSMA therapy and patient characteristics
A detailed patient characteristic is given by Table 1. Median therapy interval (including therapy pauses) was 8.2 (IQR: 3.3) weeks, median therapeutic activity was 6.2 (IQR: 1.2) GBq. The median cumulated dose was 23.7 (IQR: 25.7) GBq. Eighty percent of all patients had received taxane based chemotherapy (Docetaxel or Cabacitaxel), whereas 100% patients had received androgen deprivation therapy and 97.7% had received next generation androgen receptor targeted therapy (Enzalutamide or Abiraterone).
Descriptive statistics of baseline PET parameter measurements
The median of PSMAmax measurements was 44.6 SUV (range 7.1-181.6), whereas the median of PSMAaverage measurements was 18.9 (range 4.6-129.8). The median intensity was 31.6 (range 4.7 -159.7) for the highest SUVpeak. A detailed report on SUV parameters is provided by Table 2.
Baseline PET-parameters and overall survival
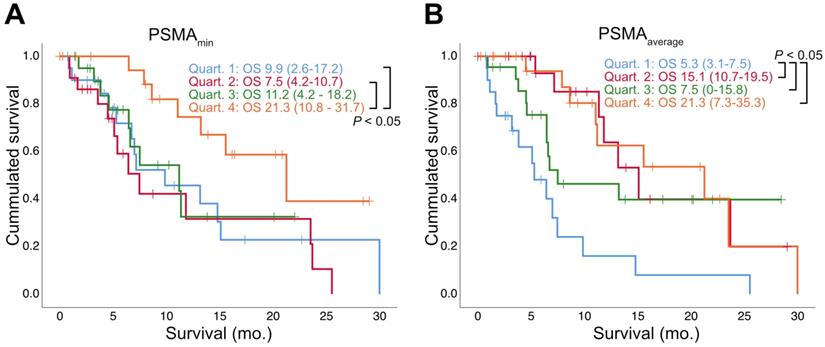
Regarding SUVmax, neither the highest (PSMAmax: HR: 0.992; p = 0.231; 95% CI: 0.979-1.005), nor the lowest (PSMAmin: HR: 0.890; p = 0.118; 95% CI: 0.768-1.030) value per patient were significant prognosticators of overall survival, but the average value was (PSMAaverage: HR: 0.959; p = 0.047; 95% CI: 0.921-0.999). The same was true for SUVmean (highest: HR= 0.989; p = 0.241; 95% CI= 0.970-1.008; average: HR = 0.941; p = 0.045; 95% CI= 0.887-0.999; lowest: HR = 0.799; p = 0.052; 95% CI = 0.638-1.002). Details (including the standard deviation of SUVmax, SUVmean and SUVpeak) are given by Table 3. Figure 1 depicts the overall survival stratified according to the quartiles of PSMAaverage and PSMAmin.
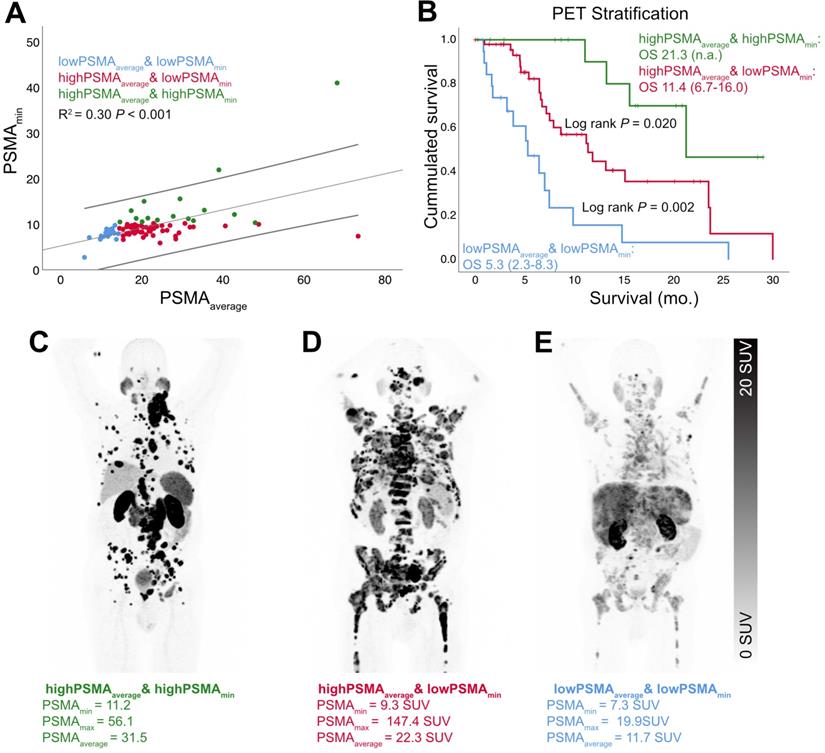
There were no relevant correlations between PSMAmin and PSMAaverage (R2 = 0.30, p < 0.001; Figure 2) or PSMAmin and PSMAstd (R2 = 0.18, p < 0.001), but PSMAstd and PSMAaverage were significantly correlated (R2 = 0.78, p < 0.001; Figure 3).
SUV parameters of the presented patient cohort (n = 85)
| PET parameter | Median of the average value of all patients | Median of the highest value of all patients | Median of the lowest value of all patients |
|---|---|---|---|
| SUVmax | 18.9 [5.9-73.4] | 44.6 [7.1-181.6] | 8.9 [2.7-40.9] |
| SUVmean | 13.0 [4.0-51.4] | 29.5 [4.6-129.8] | 6.3 [2.6-29.4] |
| SUVpeak | 12.1 [3.7-46.5] | 31.6 [4.7-159.7] | 5.7 [1.8-22.6] |
Abbreviation: Squared brackets = range.
Baseline PSMA PET parameters and overall survival
| Measurement selected per patient | HR | 95%CI | P | |
|---|---|---|---|---|
| SUVmax | Average (PSMAaverage) | 0.959 | 0.921-0.999 | 0.047 |
| Highest (PSMAmax) | 0.992 | 0.979-1.005 | 0.231 | |
| Lowest (PSMAmin) | 0.890 | 0.768-1.030 | 0.118 | |
| Std (PSMAstd) | 0.936 | 0.877-0.999 | 0.048 | |
| SUVmax / SUVmean liver | Average | 0.963 | 0.895-1.036 | 0.313 |
| Highest | 0.996 | 0.975-1.017 | 0.701 | |
| Lowest | 0.904 | 0.728-1.123 | 0.363 | |
| Std | 0.932 | 0.822-1.057 | 0.274 | |
| SUVmean | Average | 0.941 | 0.887-0.999 | 0.045 |
| Highest | 0.989 | 0.970-1.008 | 0.241 | |
| Lowest | 0.799 | 0.638-1.002 | 0.052 | |
| Std | 0.904 | 0.820-0.996 | 0.042 | |
| SUVpeak | Average | 0.941 | 0.882-1.004 | 0.064 |
| Highest | 0.989 | 0.972-1.007 | 0.227 | |
| Lowest | 0.736 | 0.533-1.016 | 0.062 | |
| Std | 0.918 | 0.844-0.999 | 0.048 |
Abbreviations: HR = Hazard ratio; CI = confidence interval; Std = standard deviation.
Overlap of PET stratification
| highPSMAaverage | lowPSMAaverage | Sum | |
|---|---|---|---|
| lowPSMAmin | 49 | 20 | 69 |
| highPSMAmin | 16 | 0 | 16 |
| Sum | 65 | 20 | 85 |
Low/highPSMAmin = patients with or without metastases that had a SUVmax above or below 10.2; high/lowPSMAaverage = patients with an average SUVmax of all metastases above or below 14.3. SUV threshold values resemble optimized log rank cut-offs.
Low PSMA expression and overall survival
In a first approach, a liver specific SUV threshold was used (1.5 × SUVmean liver) in analogy to Hofman et al. In our cohort, zero patients had a SUVmax below the liver specific threshold. Patients (n=8) with at least one tumor lesion below the liver specific SUV threshold did not have a significantly shorter overall survival time (log rank: p = 0.335; 7.5 vs.13.2 months; HR: 1.588; p = 0.340; 95% CI: 0.615-4.101).
In a second approach, PSMAaverage and PSMAmin were binarized to determine low and high PSMA expression. The optimized log rank threshold for PSMAaverage to stratify according to overall survival was 14.3 SUV (log rank: p < 0.001; estimated median: 15.1 vs. 5.3 months; high PSMAaverage: HR = 0.268, 95%CI = 0.140-0.512, p<0.001) and 10.2 for PSMAmin (log rank: p = 0.003; estimated median: 21.3 vs. 7.9 months; high PSMAmin: HR = 0.232, 95%CI = 0.082-0.658; p = 0.006). Taken together, both classifiers stratified patients into high, intermediate or low overall survival. Table 4 presents the intersection of these two classifiers; Figure 2 depicts the overall survival according to them. Patients that were classified highPSMAmin had longer survival compared to patients classified as both lowPSMAmin and highPSMAaverage (estimated median: 21.3 vs. 11.4 months; p = 0.02; HR = 0.3; 95%CI = 0.102-0.877; p = 0.028); patients classified as both lowPSMAmin and highPSMAaverage had longer survival compared to lowPSMAaverage (estimated median: 11.4 vs. 5.3 months; p = 0.002; HR = 0.364; 95%CI = 0.186-0.710; p = 0.003). Multivariate Regression (including binarized PSMAmin and lowPSMAaverage and adjusted for presence of liver metastases) confirmed both highPSMAmin and highPSMAaverage to be significant positive prognosticators of overall survival (highPSMAaverage: HR = 0.473; p = 0.044; 95%CI = 0.229-0.980 | highPSMAmin: HR = 0.300; p = 0.028; 95%CI = 0.102-0.878 | liver metastases absence: HR = 0.476; p = 0.033; 95%CI = 0.240-0.943). Optimized threshold values (both absolute and relative to liver activity) for other SUV parameters are shown by Table 5. The median volumetric fraction of low PSMA expressing tumor volume was 3.6% (IQR: 13.8) for the proposed optimized log rank threshold (10.2 SUV). The volumetric fraction of low PSMA expressing tumor volume could significantly stratify the overall survival time (>9.7% vs. =<9.7%; p = 0.023; 6.4 vs. 11.4 months; only lowPSMAmin patients).
Influence of liver metastases and Cabazitaxel therapy
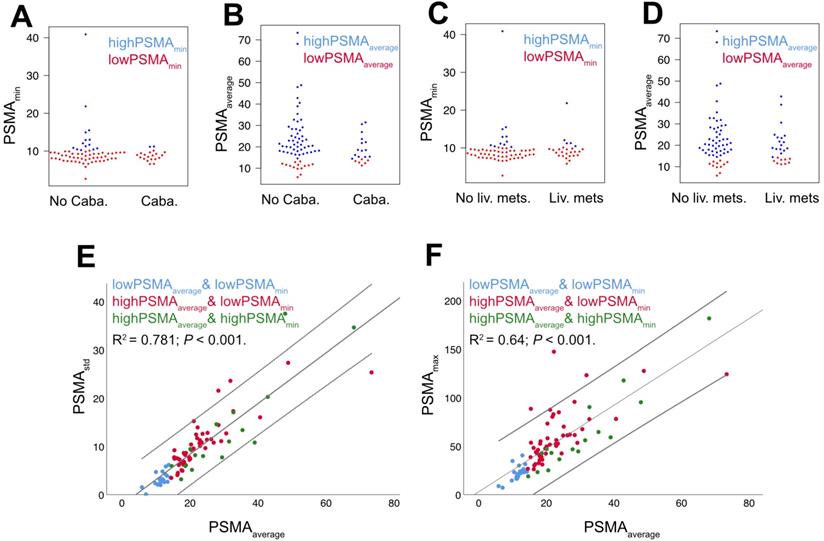
There were no significant differences between patients with and without liver metastases regarding PSMAmin (9.1vs.8.9; p = 0.418) or PSMAaverage (18.3 vs. 20.1, p = 0.264; Figure 3). The same was true for a positive/negative history of Cabazitaxel therapy (PSMAmin 8.9 vs. 9.1; p = 0.615; PSMAaverage 15.9 vs. 20.1, p = 0.138; Figure 3). There were no statistically significant associations between the presence of liver metastases and PSMAmin status (p = 1.000) or PSMAaverage status (p = 0.164). Likewise, there were no statistically significant associations between the history of Cabazitaxel therapy and PSMAmin status (p = 0.338) or PSMAaverage status (p = 0.547). In accordance with a previous study of our group, presence of liver metastases (HR = 2.775; p = 0.001; 95%CI = 1.481-5.198) as well as history of second line chemotherapy (HR = 3.047; p = 0.002; 95%CI = 1.523-6.093) were significant negative prognosticators of overall survival [18].
Discussion
The implication of low PSMA expression for the overall survival of patients treated with Lu-PSMA therapy was investigated in the present study. To this end, the correlation of overall survival and various PSMA-PET parameters was elucidated. Additionally, different PET uptake criteria have been employed to group patients into low or high PSMA expression. The highest pathological PSMA expression of a given patient under Lu-PSMA therapy (PSMAmax) could not prognosticate overall survival. Low average PSMA expression of all metastases (PSMAaverage) was associated with shorter overall survival. The absence of low PSMA expressing metastases prior to Lu-PSMA therapy was associated with best overall survival. The volumetric fraction of low PSMA expressing metastases was a negative prognosticator of overall survival.
Binarized baseline PSMA PET parameters and overall survival
| Measurement selected per patient | Ideal cut-off | P | Below cut-off | Above cut-off | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median Survival | 95%CI | n | Median Survival | 95%CI | ||||
| SUVmax | Average (=PSMAaverage) | 14.3 | <0.001 | 20 | 5.3 | 2.3-8.8 | 65 | 15.1 | 7.8-23.4 |
| Highest (=PSMAmax) | n.a. | n.s. | |||||||
| Lowest (=PSMAmin) | 10.2 | 0.003 | 69 | 7.9 | 4.6-11.2 | 16 | 21.3 | n.a. | |
| Std (=PSMAstd) | 3.0 | <0.001 | 9 | 3.2 | 0.0-6.9 | 76 | 13.2 | 9.0-17.4 | |
| SUVmax / SUVmean liver | Average | 6.2 | 0.003 | 42 | 7.2 | 5.8-8.6 | 43 | 21.3 | 13.2-29.3 |
| Highest | n.a. | n.s. | |||||||
| Lowest | n.a. | n.s. | |||||||
| Std | n.a. | n.s. | |||||||
| SUVmean | Average | 9.3 | <0.001 | 17 | 6.4 | 1.7-11.1 | 68 | 15.1 | 10.1-20.1 |
| Highest | n.a. | n.s. | |||||||
| Lowest | 7.1 | 0.004 | 65 | 8.6 | 4.3-12.9 | 20 | 25.5 | 15.1-36.0 | |
| Std | 1.9 | <0.001 | 9 | 3.2 | 0.0-6.9 | 76 | 13.2 | 9.1-17.4 | |
| SUVpeak | Average | 9.7 | <0.001 | 19 | 6.4 | 3.7-9.1 | 66 | 13.2 | 8.4-18.1 |
| Highest | n.a. | n.s. | |||||||
| Lowest | 6.6 | 0.004 | 65 | 8.6 | 4.3-12.9 | 20 | 25.5 | 15.1-36.0 | |
| Std | n.a. | n.s. | |||||||
Abbreviations: CI = confidence interval; Std = standard deviation; n.a. = not available; n.s. = not statistically significant. Bonferroni adjustment: p < 0.0125 is regarded statistically significant. Ideal cut-off was found by log rank cut-off finder.
Survival stratified by quartiles of PSMA-PET parameters. Estimated median overall survival (OS) in months (mo.) is shown together with the 95% Confidence Interval (in parentheses). Log rank test was used to compare OS between quartiles.
Survival stratified by high, intermediate and low PSMA expression. There was no relevant correlation between PSMAmin and PSMAaverage (A, linear regression and 95% CI interval). Therefore, the combination of both PET parameters enabled an optimized stratification according to overall survival (B). Exemplary patients of the high (C), intermediate (D) and low (E) overall survival group were shown additionally.
To date, there is no reasonable definition of low or high PSMA expression. However, patients in whom the PSMA expression was assessed low were not considered for Lu-PSMA therapy in the Australian Phase II trial of Hofman et al. [3,19]. Yet, the benefit of the employed PSMA expression-based inclusion criteria could not be evaluated in the very same trial. Interestingly, some patients from the present cohort that received Lu-PSMA therapy had a lower maximum PSMA expression (SUVmax 7.1) compared to the Hofman et al. cohort (SUVmax 22.1) [3]. Therefore, the present study retrospectively applied the PSMA-PET criterion of Hofman et al., to evaluate, if patients would have been judged eligible for Lu-PSMA therapy [3]. In the present cohort, the maximum PSMA expression of each patient was above the liver specific threshold of Hofman et al. (1.5 × SUVmean of liver) and therefore all would have met the inclusion criterion of Hofman et al. [3]. Eight patients had at least one metastasis with a SUVmax below this liver specific threshold. Still, there was no significant stratification of patients according to overall survival in the present cohort. Finally, in the present patient cohort, PSMAmax was not a significant prognosticator of overall survival, which is in line with a recent publication of Ferdinandus et al. [20]. Therefore, the maximum pathological PSMA expression seems unsuited to predict the therapy response of patients who show a minimum SUVmax of 7.1 at any metastatic site.
Relations of baseline PSMA-PET parameters. PSMA-PET parameters were grouped by history of Cabazitaxel chemotherapy (= Caba; A+B) or presence of liver metastases (= Liv. mets; C+D); there were no statistically significant differences. There was a high correlation of PSMAstd and PSMAaverage and a moderate correlation of PSMAmax and PSMAaverage (E+F, linear regression and 95% CI interval).
Patients with low average PSMA expression had short overall survival compared to those with high average PSMA expression and/or no low PSMA expressing metastases. Yet it remains unclear, if these patients still benefited from Lu-PSMA therapy. There is only limited evidence that the overall survival would have been worse, if Lu-PSMA therapy would not have been administered. The survival of patients with low PSMA expression that received Lu-PSMA therapy was longer in the current cohort (6.4 months) compared to patients excluded from the Lu-PSMA Phase II trial of Hofman et al. (2.5 months) [19]. Other studies have employed the survival of historic control cohorts for comparison [14]. But these comparisons might be heavily influenced by the individual metastatic spread and tumor differentiation, especially given the small patient numbers. Therefore, no rationale for PSMA-PET based exclusion criteria for Lu-PSMA can be provided by the given study. However, it seems unfavorable to use the maximum PSMA expression as decision criterion which did not correlate with overall survival.
Other prognosticators of Lu-PSMA therapy outcome have been identified in previous studies [18,21]. Amongst others, the presence of liver metastases and history of second line Cabazitaxel chemotherapy were negative prognosticators of overall survival [18,22]. Interestingly, there were no statistically significant associations between those predictors and the PSMAaverage or PSMAmin status.
Due to genetic and non-genetic variations, cancer cells both in primary tumors and metastases become heterogeneous during the course of the disease [23,24]. In prostate cancer, neuroendocrine differentiation of cancer cells may occur in advanced stages and especially after lasting androgen deprivation therapy [25-29]. Prostate cancer cell markers like prostate-specific antigen are lost during dedifferentiation, whereas neuroendocrine markers like neurone specific enolase are gained [26,30]. Neuroendocrine differentiation is generally associated with poor overall survival [31]. Interestingly, Rathke et al. could show that the neuroendocrine marker chromogranin A is a moderately negative predictor for response in patients treated by Lu-PSMA therapy [12]. In the present study, the average PSMA expression was a positive prognosticator for overall survival. Interestingly, average PSMA expression and variation of PSMA uptake (measured as PSMAstd) between metastases were highly correlated. Both findings might be partly explained by dedifferentiation of prostate cancer cells in metastases. This might be contradictory, as one could assume that decreased average PSMA expression is associated with more heterogeneity between metastases (i.e. side by side presence of metastases with low and with high PSMA expression). However, it remains unclear if the shorter overall survival of patients with low PSMA expressing metastases is due to dedifferentiated and more aggressive tumor phenotypes, or due to reduced efficacy of Lu-PSMA therapy.
PSMA PET can visualize PSMA expression in the living patient and Lu-PSMA therapy targets PSMA expressing cells. Therefore, it has been hypothesized that PSMA PET can predict the achieved radiation dose, which is deposited in the tumor, and thereby indirectly predict the treatment response. The work of Violet et al. had shown that the mean whole body PSMA PET uptake indeed correlated with the absorbed doses of Lu-PSMA therapy [32]. Moreover, patients that obtained doses <10 Gy had unfavorable PSA response rates [32]. However, no survival data was studied by Violet et al. [32]. In contrast, the present manuscript could show that mean whole body PSMA expression is a significant predictor of survival.
The overall survival of patients that present strong PSMA expressing metastases, but likewise have less PSMA expressing metastases is currently unclear. Interestingly, highPSMAmin patients had a significantly longer overall survival. Taken together with PSMAaverage, patients were stratified based on PSMA-PET measurements into those with low (lowPSMAaverage), intermediate (highPSMAaverage and lowPSMAmin) or high (highPSMAmin) PSMA expression. Thereby, patients could be stratified in those with good, intermediate and poor overall survival. This is depicted by Figure 2. Moreover, the volumetric fraction of low PSMA expressing metastases was a negative prognosticator for overall survival. Future studies have to elucidate, if the occurrence of low PSMA expressing metastases (i.e. lowPSMAmin) is sequentially followed by the tendency to PSMA decrease in all metastases (i.e. lowPSMAaverage) in the process of the disease. Moreover, it might be warranted to elucidate, if this sequence is caused by occurrence and spared of dedifferentiated tumor cell phenotypes.
The present study has limitations. It was conducted retrospectively and is therefore prone to selection biases. The patient collective might not be comparable to the Phase II trial of Hofman et al. or other retrospective analyses [13]. However, retrospective studies are mandatory for the planning of prospective trials, especially for inclusion criteria definition. In the present analysis, no FDG PET-CT was employed for patient selection. Therefore, PSMA negative metastases that show strong FDG uptake might have evaded the analysis. Additionally, small metastases (<0.5 ml) were no considered in the analysis. The SUVmean heavily depends on the segmentation method. In contrast to previous approaches, we did not use a fixed SUV threshold (e.g. SUV > 3) but relative thresholding (50% of local SUVmax). Therefore, SUVmean results are not directly comparable with other studies. Because of that, only SUVmax based PET parameters (PSMAmax, PSMAaverage, PSMAmin, PSMAstd) were used for hypotheses generation.
Conclusion
In this retrospective analysis, PSMAaverage was a significant prognosticator for overall survival of mCRPC patients treated with Lu-PSMA therapy, whereas PSMAmax was not. Patients without low PSMA expressing metastases had the best overall survival. Future studies are warranted to elucidate the degree of low PSMA expression tolerable for Lu-PSMA therapy.
Abbreviations
PET: Positron Emission Tomography; PSMA: Prostate Specific Membrane Antigen.
Acknowledgements
We acknowledge support from the Open Access Publication Fund of the University of Muenster.
Compliance with Ethical Standards
Disclosure of potential conflicts of interest
KR has received consultant fees from Bayer and lectureship fees from Janssen Cilag, Amgen, AAA and SIRTEX. KR is a clinical consultant for ABX. The University of Muenster has received consulting fees from ABX Advanced Biochemical Compounds, Radeberg, Germany for KR. MB has received consultant and lectureship fees from Bayer, Janssen Cilag, Astellas, ABX, Sanofi, Eisai, EUSApharm, Pfizer, BMS, MSD, AstraZeneca, Merck, Amgen, Novartis, Exelixis and Roche. The authors declare that they have no conflict of interest regarding this study.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Data analysis was done retrospectively and was approved by the local ethics committee (No. 2016-585-f-S, Ethikkommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster).
Informed consent
Lu-PSMA therapy was done on a case by case basis in the clinical routine and only after recommendation by the local tumor board. Informed consent for the therapy was present for all patients.
References
1. Sartor O, de Bono JS. Metastatic Prostate Cancer. N Engl J Med. 2018;378:645-57
2. Rahbar K, Bögeman M, Yordanova A. et al. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:243-6
3. Violet J, Sandhu S, Iravani A. et al. Long term follow-up and outcomes of re-treatment in an expanded 50 patient single-center phase II prospective trial of Lutetium-177 (177Lu) PSMA-617 theranostics in metastatic castrate-resistant prostate cancer. J Nucl Med. 2019 10.2967/jnumed.119.236414
4. Rahbar K, Ahmadzadehfar H, Kratochwil C. et al. German Multicenter Study Investigating 177 Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med. 2017;58:85-90
5. Donin NM, Reiter RE. Why Targeting PSMA Is a Game Changer in the Management of Prostate Cancer. J Nucl Med. 2018;59:177-82
6. Virgolini I, Decristoforo C, Haug A, Fanti S, Uprimny C. Current status of theranostics in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:471-95
7. Zamboglou C, Schiller F, Fechter T. et al. 68 Ga-HBED-CC-PSMA PET/CT Versus Histopathology In Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics. 2016;6:1619-28
8. Zamboglou C, Drendel V, Jilg CA. et al. Comparison of 68 Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7:228-37
9. Zamboglou C, Carles M, Fechter T. et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer - a comparison study with histology reference. Theranostics. 2019;9:2595-605
10. Kratochwil C, Fendler WP, Eiber M. et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2019;46:2536-44
11. Ferdinandus J, Eppard E, Gaertner FC. et al. Predictors of Response to Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with 177 Lu-PSMA-617. J Nucl Med. 2017;58:312-9
12. Rathke H, Holland-Letz T, Mier W. et al. Response Prediction of 177 Lu-PSMA-617 Radioligand Therapy Using Prostate-Specific Antigen, Chromogranin A, and Lactate Dehydrogenase. J Nucl Med. 2020;61:689-95
13. Hofman MS, Violet J, Hicks RJ. et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825-33
14. Rahbar K, Bode A, Weckesser M. et al. Radioligand Therapy With 177Lu-PSMA-617 as A Novel Therapeutic Option in Patients With Metastatic Castration Resistant Prostate Cancer. Clin Nucl Med. 2016;41:522-8
15. Seifert R, Herrmann K, Kleesiek J. et al. Semi-automatically quantified tumor volume using Ga-68-PSMA-11-PET as biomarker for survival in patients with advanced prostate cancer. J Nucl Med. 2020 10.2967/jnumed.120.242057
16. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2018
17. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43:121-37
18. Kessel K, Seifert R, Schäfers M. et al. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177 Lu-PSMA-617. Theranostics. 2019;9:4841-8
19. Thang SP, Violet J, Sandhu S. et al. Poor Outcomes for Patients with Metastatic Castration-resistant Prostate Cancer with Low Prostate-specific Membrane Antigen (PSMA) Expression Deemed Ineligible for 177Lu-labelled PSMA Radioligand Therapy. Eur Urol Oncol. 2019;2:670-6
20. Ferdinandus J, Violet J, Sandhu S. et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur J Nucl Med Mol Imaging. 2020:6-11
21. Ahmadzadehfar H, Schlolaut S, Fimmers R. et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [177Lu]Lu-PSMA-617 radioligand therapy. Oncotarget. 2017;8:103108-16
22. von Eyben FE, Kulkarni HR, Baum RP. Metastatic extent predicts survival as patients with metastatic castration-resistant prostate cancer are treated with 177 Lu-PSMA radioligand therapy. Theranostics. 2020;10:4900-2
23. Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323-34
24. Gerlinger M, Rowan AJ, Horswell S. et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med. 2012;366:883-92
25. Pouessel D, Gallet B, Bibeau F. et al. Liver metastases in prostate carcinoma: Clinical characteristics and outcome. BJU Int. 2007;99:807-11
26. Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: Neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531-47
27. Molenaar JPF, Baten A, Blokx WAM, Hoogendam A. Development of Carcinoid Tumour in Hormonally Treated Adenocarcinoma of the Prostate. Eur Urol. 2009;56:874-7
28. Wang HT, Yao YH, Li BG, Tang Y, Chang JW, Zhang J. Neuroendocrine Prostate Cancer (NEPC) Progressing from conventional prostatic adenocarcinoma: Factors associated with time to development of nepc and survival from NEPC Diagnosis-A systematic review and pooled analysis. J Clin Oncol. 2014;32:3383-90
29. Conteduca V, Oromendia C, Eng KW. et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer. 2019;121:7-18
30. Bakht MK, Derecichei I, Li Y. et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr Relat Cancer. 2019;26:131-46
31. Aggarwal R, Huang J, Alumkal JJ. et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J Clin Oncol. 2018;36:2492-503
32. Violet J, Jackson P, Ferdinandus J. et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: Correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med. 2019;60:517-23
Author contact
![]() Corresponding author: Kambiz Rahbar, MD, Department of Nuclear Medicine; University Hospital Muenster, Albert-Schweitzer-Campus 1, D-48149 Münster, Germany. Tel.: +49-251-8347362; Fax: +49-251-8347363; E-mail: rahbarde.
Corresponding author: Kambiz Rahbar, MD, Department of Nuclear Medicine; University Hospital Muenster, Albert-Schweitzer-Campus 1, D-48149 Münster, Germany. Tel.: +49-251-8347362; Fax: +49-251-8347363; E-mail: rahbarde.
 Global reach, higher impact
Global reach, higher impact