13.3
Impact Factor
Theranostics 2020; 10(18):8264-8280. doi:10.7150/thno.45537 This issue Cite
Research Paper
A new class of PentixaFor- and PentixaTher-based theranostic agents with enhanced CXCR4-targeting efficiency
1. Chair for Pharmaceutical Radiochemistry, Faculties of Chemistry and Medicine, Technische Universität München, Garching, Germany.
2. Department of Nuclear Medicine, Klinikum rechts der Isar, Technische Universität München, Munich, Germany.
3. Translational Radiopharmaceutical Sciences, Departments of Nuclear Medicine and of Oncology, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland.
*These authors contributed equally to this work.
Abstract
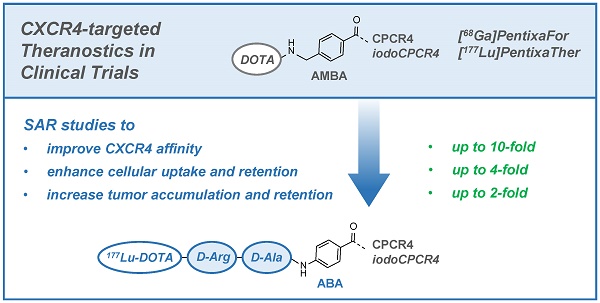
Non-invasive PET imaging of CXCR4 expression in cancer and inflammation as well as CXCR4-targeted radioligand therapy (RLT) have recently found their way into clinical research by the development of the theranostic agents [68Ga]PentixaFor (cyclo(D-Tyr1-D-[NMe]Orn2(AMBS-[68Ga]DOTA)-Arg3-Nal4-Gly5) = [68Ga]DOTA-AMBS-CPCR4) and [177Lu/90Y]PentixaTher (cyclo(D-3-iodo-Tyr1-D-[NMe]Orn2(AMBS-[177Lu/90Y]DOTA)-Arg3-Nal4-Gly5) = [177Lu/90Y]DOTA-AMBS-iodoCPCR4). Although convincing clinical results have already been obtained with both agents, this study was designed to further investigate the required structural elements for improved ligand-receptor interaction for both peptide cores (CPCR4 and iodoCPCR4). To this aim, a series of DOTA-conjugated CPCR4- and iodoCPCR4-based ligands with new linker structures, replacing the AMBA-linker in PentixaFor and PentixaTher, were synthesized and evaluated.
Methods: The in vitro investigation of the novel compounds alongside with the reference peptides PentixaFor and PentixaTher encompassed the determination of hCXCR4 and mCXCR4 affinity (IC50) of the respective natGa-, natLu-, natY- and natBi-complexes in Jurkat and Eμ-myc 1080 cells using [125I]FC-131 and [125I]CPCR4.3 as radioligands, respectively, as well as the evaluation of the internalization and externalization kinetics of selected 68Ga- and 177Lu-labeled compounds in hCXCR4-transfected Chem-1 cells. Comparative small animal PET imaging studies (1h p.i.) as well as in vivo biodistribution studies (1, 6 and 48h p.i.) were performed in Daudi (human B cell lymphoma) xenograft bearing CB17 SCID mice.
Results: Based on the affinity data and cellular uptake studies, [68Ga/177Lu]DOTA-r-a-ABA-CPCR4 and [68Ga/177Lu]DOTA-r-a-ABA-iodoCPCR4 (with r-a-ABA = D-Arg-D-Ala-4-aminobenzoyl-) were selected for further evaluation. Both analogs show app. 10-fold enhanced hCXCR4 affinity compared to the respective references [68Ga]PentixaFor and [177Lu]PentixaTher, four times higher cellular uptake in hCXCR4 expressing cells and improved cellular retention. Unfortunately, the improved in vitro binding and uptake characteristics of [68Ga]DOTA-r-a-ABA-CPCR4 and -iodoCPCR4 could not be recapitulated in initial PET imaging studies; both compounds showed similar uptake in the Daudi xenografts as [68Ga]PentixaFor, alongside with higher background accumulation, especially in the kidneys. However, the subsequent biodistribution studies performed for the corresponding 177Lu-labeled analogs revealed a clear superiority of [177Lu]DOTA-r-a-ABA-CPCR4 and [177Lu]DOTA-r-a-ABA-iodoCPCR4 over [177Lu]PentixaTher with respect to tumor uptake (18.3±3.7 and 17.2±2.0 %iD/g, respectively, at 1h p.i. vs 12.4±3.7%iD/g for [177Lu]PentixaTher) as well as activity retention in tumor up to 48h. Especially for [177Lu]DOTA-r-a-ABA-CPCR4 with its low background accumulation, tumor/organ ratios at 48h were 2- to 4-fold higher than those obtained for [177Lu]PentixaTher (except for kidney).
Conclusions: The in-depth evaluation of a series of novel CPCR4- and iodoCPCR4 analogs with modified linker structure has yielded reliable structure-activity relationships. It was generally observed that a) AMBA-by-ABA-substitution leads to enhanced ligand internalization, b) the extension of the ABA-linker by two additional amino acids (DOTA-Xaa2-Xaa1-ABA-) provides sufficient linker length to minimize the interaction of the [M3+]DOTA-chelate with the receptor, and that c) introduction of a cationic side chain (Xaa2) greatly enhances receptor affinity of the constructs, obliterating the necessity for Tyr1-iodination of the pentapeptide core to maintain high receptor affinity (such as in [177Lu]PentixaTher).
As a result, [177Lu]DOTA-r-a-ABA-CPCR4 has emerged from this study as a powerful second-generation therapeutic CXCR4 ligand with greatly improved targeting efficiency and tumor retention and will be further evaluated in preclinical and clinical CXCR4-targeted dosimetry and RLT studies.
Keywords: CXCR4, cyclic pentapeptide, PET, radioligand therapy, cancer
 Global reach, higher impact
Global reach, higher impact