13.3
Impact Factor
Theranostics 2020; 10(18):8382-8399. doi:10.7150/thno.45391 This issue Cite
Research Paper
Nanomicelle protects the immune activation effects of Paclitaxel and sensitizes tumors to anti-PD-1 Immunotherapy
1. State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, 610041, P.R. China.
2. School of Pharmaceutical Science & Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming, Yunnan, 650500, P.R. China.
3. Department of Obstetrics, Sichuan Provincial Hospital for Women and Children, Chengdu, Sichuan, P.R. China.
4. Department of medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
5. Innovent Biologics, Inc., Suzhou, Jiangsu, P.R. China.
6. Guangdong Zhongsheng Pharmaceutical Co., Ltd. China.
*Contributed equally to this work as first authors.
Abstract
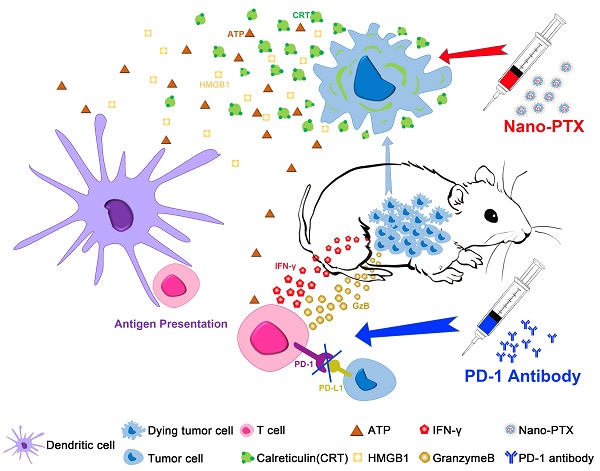
Paclitaxel (PTX) has shown pleiotropic immunologic effects on the tumor microenvironment, and nanomicelle has emerged as a promising strategy for PTX delivery. However, the detailed mechanisms remain to be fully elucidated. Meanwhile, immunogenic cell death (ICD) is an effective approach to activate the immune system. This study investigated the ICD effect of PTX and how nanomicelle affected the immune-activation ability of PTX.
Methods: The ICD effects of PTX were identified via the expression of ICD markers and cell vaccine experiment. Tumor size and overall survival in multiple animal models with treatment were monitored to evaluate the antitumor effects. The mechanisms of PTX-induced ICD and antitumor immunity were determined by detecting gene expression related to ER stress and analyzing immune cell profile in tumor after treatment.
Results: We revealed the immune-regulation mechanism of PTX nanomicelle by inducing ICD, which can promote antigen presentation by dendritic cells (DCs) and activate antitumor immunity. Notably, nanomicelle encapsulation protected the ICD effects and immune activation, which were hampered by immune system impairment caused by chemotherapy. Compared with traditional formulations, a low dose of nanomicelle-encapsulated PTX (nano-PTX) treatment induced immune-dependent tumor control, which increased the infiltration and function of both T cells and DCs within tumors. However, this antitumor immunity was hampered by highly expressed PD-1 on tumor-infiltrating CD8+ T cells and upregulated PD-L1 on both immune cells and tumor cells after nano-PTX treatment. Combination therapy with a low dose of nano-PTX and PD-1 antibodies elicited CD8+ T cell-dependent antitumor immunity and remarkably improved the therapeutic efficacy.
Conclusions: Our results provide systemic insights into the immune-regulation ability of PTX to induce ICD, which acts as an inducer of endogenous vaccines through ICD effects, and also provides an experimental basis for clinical combination therapy with nano-PTX and PD-1 antibodies.
Keywords: Paclitaxel (PTX), Nanomicelle, Immunogenic cell death (ICD), anti-PD-1 immunotherapy, Combination immunotherapy
 Global reach, higher impact
Global reach, higher impact