13.3
Impact Factor
Theranostics 2020; 10(19):8606-8618. doi:10.7150/thno.46861 This issue Cite
Research Paper
Fructose-1, 6-bisphosphatase 1 interacts with NF-κB p65 to regulate breast tumorigenesis via PIM2 induced phosphorylation
1. Department of Reproductive Medicine, Affiliated Hospital of Weifang Medical University, Weifang, Shandong Province, P.R. China.
2. Department of Pathology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong Province, P.R. China.
*These authors contributed equally to this work.
Abstract
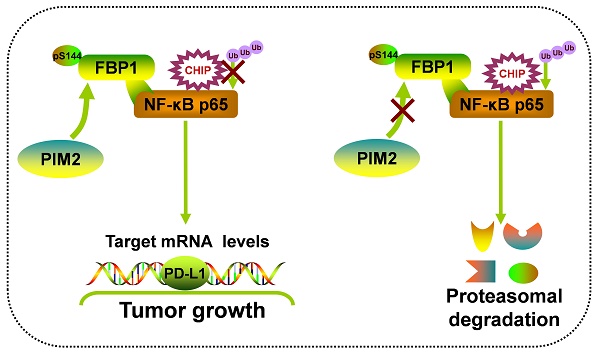
Rationale: Fructose-1, 6-bisphosphatase 1 (FBP1), a rate-limiting enzyme in gluconeogenesis, was recently shown to be a tumor suppressor and could mediate the activities of multiple transcriptional factors via its non-canonical functions. However, the underlying mechanism of posttranscriptional modification on the non-canonical functions of FBP1 remains elusive.
Methods: We employed immunoaffinity purification to identify binding partner(s) and used co-immunoprecipitation to verify their interactions. Kinase reaction was used to confirm PIM2 could phosphorylate FBP1. Overexpression or knockdown proteins were used to assess the role in modulating p65 protein stability. Mechanistic analysis was involved in protein degradation and polyubiquitination assays. Nude mice and PIM2-knockout mice was used to study protein functions in vitro and in vivo.
Results: Here, we identified Proviral Insertion in Murine Lymphomas 2 (PIM2) as a new binding partner of FBP1, which could phosphorylate FBP1 on Ser144. Surprisingly, phosphorylated FBP1 Ser144 abrogated its interaction with NF-κB p65, promoting its protein stability through the CHIP-mediated proteasome pathway. Furthermore, phosphorylation of FBP1 on Ser144 increased p65 regulated PD-L1 expression. As a result, phosphorylation of FBP1 on Ser144 promoted breast tumor growth in vitro and in vivo. Moreover, the levels of PIM2 and pSer144-FBP1 proteins were positively correlated with each other in human breast cancer and PIM2 knockout mice.
Conclusions: Our findings revealed that phosphorylation noncanonical FBP1 by PIM2 was a novel regulator of NF-κB pathway, and highlights PIM2 inhibitors as breast cancer therapeutics.
Keywords: PIM2, FBP1, phosphorylation, protein stability, tumor growth
 Global reach, higher impact
Global reach, higher impact