13.3
Impact Factor
Theranostics 2020; 10(20):8974-8995. doi:10.7150/thno.44912 This issue Cite
Research Paper
Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p
1. Department of Orthopedics, Xijing Hospital, the Fourth Military Medical University, Xi'an, 710032, People's Republic of China.
2. Department of Orthopedics, the General Hospital of Central Theater Command of People's Liberation Army, Wuhan, 430070, People's Republic of China.
3. Department of Spine Surgery, Honghui Hospital Affiliated to Medical School of Xi'an Jiaotong University, Xi'an Shaanxi, 710054, People's Republic of China.
4. Department of Orthopedics, the 985th Hospital People's Liberation Army Joint Logistics Support Force, Taiyuan, 030000, People's Republic of China.
5. Department of Spinal Surgery, the People's Hospital of Longhua District, Shenzhen, 518109, People's Republic of China.
6. Department of Oral Anatomy and Physiology, State Key Laboratory of Military Stomatology & National Clinical Research Center for Oral Diseases & Shaanxi Key Laboratory of Stomatology, School of Stomatology, the Fourth Military Medical University, Xi'an, 710032, People's Republic of China.
*These authors contributed equally to this work.
Abstract
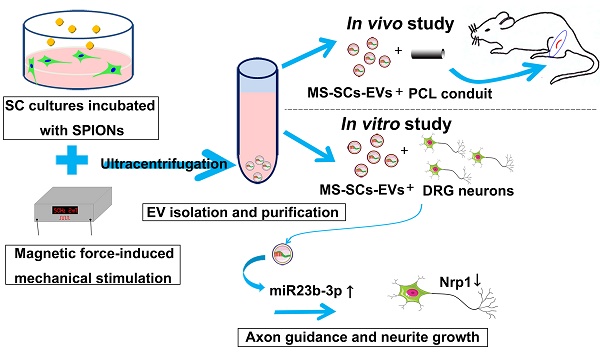
Rationale: Peripheral nerves are unique in their remarkable elasticity. Schwann cells (SCs), important components of the peripheral nervous system (PNS), are constantly subjected to physiological and mechanical stresses from dynamic stretching and compression forces during movement. So far, it is not clear if SCs sense and respond to mechanical signals. It is also unknown whether mechanical stimuli can interfere with the intercellular communications between neurons and SCs, and what role extracellular vesicles (EVs) play in this process. The present study aimed to examine the effect of mechanical stimuli on the EV-mediated intercellular communication between neurons and SCs, explore their effect on axonal regeneration, and investigate the underlying mechanism.
Methods: Purified SCs were stimulated using a magnetic force-based mechanical stimulation (MS) system and EVs were purified from mechanically stimulated SCs (MS-SCs-EVs) and non-stimulated SCs (SCs-EVs). The effect of MS-SCs-EVs on axonal elongation was examined in vitro and in vivo. High throughput miRNA sequencing was performed to compare the differential miRNA profiles between MS-SCs-EVs and SCs-EVs. The functional role of differentially expressed miRNAs on neurite extension in MS-SCs-EVs was examined. Also, the putative target genes of differentially expressed miRNAs in MS-SCs-EVs were predicted by bioinformatics tools, and the regulatory effect of those miRNAs on putative target genes was validated both in vitro and in vivo.
Results: The MS-SCs-EVs showed an average size of 137.52±1.77 nm, and could be internalized by dorsal root ganglion (DRG) neurons. Compared to SCs-EVs, MS-SCs-EVs showed a stronger ability to enhance neurite outgrowth in vitro and nerve regeneration in vivo. High throughput miRNA sequencing identified a number of differentially expressed miRNAs in MS-SCs-EVs. Further analysis of those EV-miRNAs demonstrated that miR-23b-3p played a predominant role in MS-SCs-EVs since its deprivation abolished their enhanced axonal elongation. Furthermore, we identified neuropilin 1 (Nrp1) in neurons as the target gene of miR-23b-3p in MS-SCs-EVs. This observation was supported by the evidence that miR-23b-3p could decrease Nrp1-3'-UTR-WT luciferase activity in vitro and down-regulate Nrp1 expression in neurons.
Conclusion: Our findings suggested that mechanical stimuli are capable of modulating the intercellular communication between neurons and SCs by altering miRNA composition in MS-SCs-EVs. Transfer of miR-23b-3p by MS-SCs-EVs from mechanically stimulated SCs to neurons decreased neuronal Nrp1 expression, which was responsible, at least in part, for the beneficial effect of MS-SCs-EVs on axonal regeneration. Our results highlighted the potential therapeutic value of MS-SCs-EVs and miR-23b-3p-enriched EVs in peripheral nerve injury repair.
Keywords: Schwann cell, mechanical stimuli, extracellular vesicle, nerve regeneration, miRNA 23b-3p
 Global reach, higher impact
Global reach, higher impact