13.3
Impact Factor
Theranostics 2020; 10(21):9808-9829. doi:10.7150/thno.43631 This issue Cite
Research Paper
Efficacy-shaping nanomedicine by loading Calcium Peroxide into Tumor Microenvironment-responsive Nanoparticles for the Antitumor Therapy of Prostate Cancer
1. Department of Urology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 20092, China.
2. Department of Urology, Huadong Hospital Affiliated to Fudan University, Shanghai 20040, China.
*These authors contributed equally to this work.
Abstract
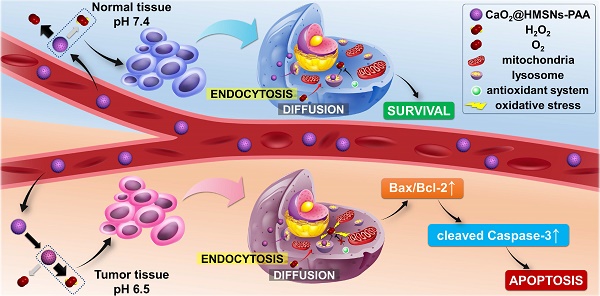
Rationale: Prostate cancer has become one of the most threatening malignant tumors in men, leading to an imperative need to develop effective and safe therapies. Because of the unique metabolism of tumor cells, the tumor microenvironment (TME) exhibits distinctive properties compared with normal tissues, among which the pH difference has been utilized as an ideal antitumor strategy. Herein, we introduce a reactive oxygen species (ROS)-controlled-release nanosystem with TME-responsiveness by applying hollow mesoporous silica nanoparticles (HMSNs) as carriers loaded with calcium peroxide (CaO2) and coated with polyacrylic acid (PAA) to construct the functional material CaO2@HMSNs-PAA. The differences in pH values and exogenous ROS scavenging abilities between the tumor tissue and normal tissues and the dual pH-responsiveness from CaO2 and PAA lay a scientific foundation for the application of CaO2@HMSNs-PAA in the tumor-selective therapy for prostate cancer.
Methods: The morphology and the structure of the nanosystem were characterized by the transmission electron microscope, scanning electron microscope, energy-dispersive X-ray spectroscopy, Fourier transform infrared spectroscopy, zeta potential, dynamic light scattering measurement, low-angle X-ray diffraction patterns and nitrogen adsorption/desorption isotherm. The CaO2 loading capacity and release profiles in different buffer solutions were determined by inductively coupled plasma-mass spectrometry. The in vitro intracellular uptake of CaO2@HMSNs-PAA was explored on the PC-3 prostate cancer cell line via confocal laser scanning microscopy. The CCK-8 cell proliferation assay was conducted to evaluate the cytotoxicity of CaO2@HMSNs-PAA against PC-3 cells. ROS produced by CaO2@HMSNs-PAA was observed by a fluorescence microscope. The flow cytometry was utilized to analyze the apoptosis of PC-3 cells induced by CaO2@HMSNs-PAA. The Western blot analysis was performed to detect expressions of critical mitochondria-mediated apoptosis markers in PC-3 cells after incubation with CaO2@HMSNs-PAA. The in vivo biosafety and antitumor efficacy were evaluated out on BALB/c mice and BALB/c nude mice subcutaneously transplanted with PC-3 cells, respectively.
Results: Comprehensive characterizations indicated the successful synthesis of CaO2@HMSNs-PAA with significant TME-responsiveness. The experimental results demonstrated that the well-developed nanocarrier could efficiently deliver CaO2 to the tumor site and release ROS in response to the decreased pH value of TME, exerting ideal antitumor effects both in vitro and in vivo by activating the mitochondria-mediated apoptosis pathway. Simultaneously, this nanoplatform caused no detectable damage to normal tissues.
Conclusions: After loading into the above nanocomposite, the free CaO2 without a significant antitumor effect can exert excellent antitumor efficacy by responsively releasing ROS under the acidic TME to induce the mitochondria-mediated apoptosis via remarkable oxidative stress and simultaneously minimize damages to normal tissues. The current study presents a new concept of “efficacy-shaping nanomedicine” for the tumor-selective treatment of prostate cancer.
Keywords: calcium peroxide, hollow mesoporous silica nanoparticles, prostate cancer, reactive oxygen species, tumor microenvironment
 Global reach, higher impact
Global reach, higher impact