13.3
Impact Factor
Theranostics 2021; 11(1):48-63. doi:10.7150/thno.50794 This issue Cite
Research Paper
A skeleton muscle model using GelMA-based cell-aligned bioink processed with an electric-field assisted 3D/4D bioprinting
1. Department of Biomechatronic Engineering, College of Biotechnology and Bioengineering, Sungkyunkwan University, Suwon 16419, Republic of Korea.
2. Biomedical Institute for Convergence at SKKU, Sungkyunkwan University, Suwon 16419, Republic of Korea.
#These authors contributed equally to this work.
Abstract
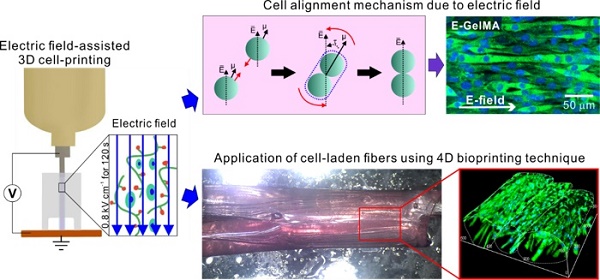
The most important requirements of biomedical substitutes used in muscle tissue regeneration are appropriate topographical cues and bioactive components for the induction of myogenic differentiation/maturation. Here, we developed an electric field-assisted 3D cell-printing process to fabricate cell-laden fibers with a cell-alignment cue.
Methods: We used gelatin methacryloyl (GelMA) laden with C2C12 cells. The cells in the GelMA fiber were exposed to electrical stimulation, which induced cell alignment. Various cellular activities, such as cell viability, cell guidance, and proliferation/myogenic differentiation of the microfibrous cells in GelMA, were investigated in response to parameters (applied electric fields, viscosity of the bioink, and encapsulated cell density). In addition, a cell-laden fibrous bundle mimicking the structure of the perimysium was designed using gelatin hydrogel in conjunction with a 4D bioprinting technique.
Results: Cell-laden microfibers were fabricated using optimized process parameters (electric field intensity = 0.8 kV cm-1, applying time = 12 s, and cell number = 15 × 106 cells mL-1). The cell alignment induced by the electric field promoted significantly greater myotube formation, formation of highly ordered myotubes, and enhanced maturation, compared to the normally printed cell-laden structure. The shape change mechanism that involved the swelling properties and folding abilities of gelatin was successfully evaluated, and we bundled the GelMA microfibers using a 4D-conceptualized gelatin film.
Conclusion: The C2C12-laden GelMA structure demonstrated effective myotube formation/maturation in response to stimulation with an electric field. Based on these results, we propose that our cell-laden fibrous bundles can be employed as in vitro drug testing models for obtaining insights into the various myogenic responses.
Keywords: GelMA, cell-laden structure, electrical stimulation, muscle, in vitro model
 Global reach, higher impact
Global reach, higher impact