13.3
Impact Factor
Theranostics 2021; 11(1):257-267. doi:10.7150/thno.51243 This issue Cite
Research Paper
Utilization of circulating cell-free DNA profiling to guide first-line chemotherapy in advanced lung squamous cell carcinoma
1. Department of Medical Oncology, Shanghai Pulmonary Hospital, Thoracic Cancer Institute, Tongji University School of Medicine, Shanghai, China.
2. Department of Respiration, Shanghai Chest Hospital, Shanghai, China.
3. Cancer Center, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
4. Department of Medical Oncology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China.
5. Department of Chemotherapy, Anhui Provincial Hospital, Hefei, Anhui, China.
6. Department of Oncology, Fuzhou Pulmonary Hospital of Fujian, Fuzhou, Fujian, China.
7. Department of Respiration, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China.
8. Department of Respiration, Henan Cancer Hospital, Zhengzhou, Henan, China.
9. Medical Oncology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China.
10. Department of Medical Oncology, Hunan Cancer Hospital, Changsha, China.
11. Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Wenzhou Medical College, Wenzhou, Zhejiang, China.
12. Department of Medical Oncology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China.
13. Department of Oncology, Xiang Yang Central Hospital, Xiangyang, Hubei, China.
14. Department of Oncology, Renmin Hospital of Wuhan University, Wuhan, Hubei, China.
15. Department of Respiration, General Hospital of Eastern Theater Command of Chinese People's Liberation Army, Nanjing, Jiangsu, China.
16. Department of Medical Oncology, Zhejiang Cancer Hospital, Hangzhou, China.
17. Cancer Center, The First Bethune Hospital of Jilin University, Changchun, Jilin, China.
18. Department of Oncology, Harbin Medical University Cancer Hospital, Harbin, China.
19. Department of Respiratory Medicine, Weifang People's Hospital, Weifang, Shandong, China.
20. Medical Oncology, Affiliated Hospital of Hebei University, Baoding, Hebei, China.
21. Department of Internal Medicine, Shandong Cancer Hospital & Institute, Jinan, Shandong, China.
22. Department of Thoracic Surgery, The Third Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China.
23. Medical Oncology, Beijing Cancer Hospital, Beijing, China.
24. Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China.
25. Department of Chemotherapy, Sichuan Cancer Hospital & Institute, Chengdu, Sichuan, China.
26. Department of Oncology, The Fourth People's Hospital of Wuxi, Wuxi, Jiangsu, China.
27. Medical Oncology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China.
28. Department of Medical Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China.
29. Department of Oncology, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China.
30. Department of Oncology, Qingdao Municipal Hospital, Qingdao, Shandong, China.
31. Medical Oncology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China.
32. Department of Respiration, Peking University First Hospital, Beijing, China.
33. Department of Thoracic Surgery, 4th Hospital of Hebei Medical University, Shijiazhuang, Hebei, China.
34. Department of Oncology, Weifang People's Hospital, Weifang, Shandong, China.
35. Medical Oncology, Cancer Hospital Chinese Academy of Medical Sciences, Beijing, China.
36. Beijing Genecast Biotechnology Co., Beijing, China.
37. Nanjing Luye Pharmaceutical Co. Ltd, Nanjing, Jiangsu, China.
#These authors contributed equally to this work.
Abstract
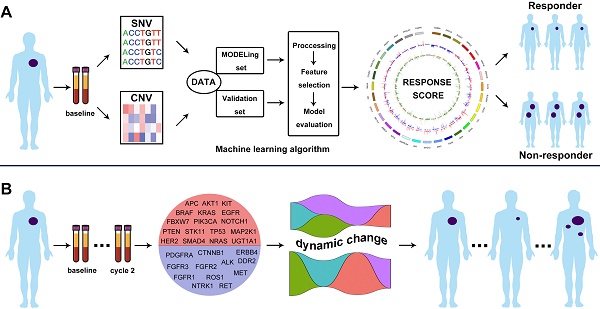
Rationale: Platinum-based chemotherapy is one of treatment mainstay for patients with advanced lung squamous cell carcinoma (LUSC) but it is still a “one-size fits all” approach. Here, we aimed to investigate the predictive and monitoring role of circulating cell-free DNA (cfDNA) profiling for the outcome of first-line chemotherapy in patients with advanced LUSC.
Methods: Peripheral blood samples of 155 patients from a phase IV trial and 42 cases from an external real-world cohort were prospectively collected. We generated a copy number variations-based classifier via machine learning algorithm to integrate molecular profiling of cfDNA, named RESPONSE SCORE (RS) to predict the treatment outcome. To monitor the treatment efficacy, cfDNA samples collected at different time points were subjected to an ultra-deep sequencing platform.
Results: The results showed that patients with high RS showed substantially higher objective response rate than those with low RS in training set (P < 0.001), validation set (P < 0.001) and real-world cohort (P = 0.019). Furthermore, a significant difference was observed in both progression-free survival (training set, P < 0.001; validation set: P < 0.001; real-world cohort: P = 0.019) and overall survival (training set, P < 0.001; validation set: P = 0.037) between high and low RS group. Notably, variant allele frequency (VAF) calculated from an ultra-deep sequencing platform significantly reduced in patients experienced a complete or partial response after 2 cycles of chemotherapy (P < 0.001), while it significantly increased in these of non-responder (P < 0.001). Moreover, VAF undetectable after 2 cycles of chemotherapy was correlated with markedly better objective response rate (P < 0.001) and progression-free survival (P < 0.001) than those with detectable VAF.
Conclusions: These findings indicated that the RS, a circulating cfDNA sequencing-based stratification index, could help to guide first-line chemotherapy in advanced LUSC. The change of VAF is valuable to monitor the treatment response.
Keywords: Non-small-cell lung cancer, cell-free DNA, chemotherapy, machine learning
 Global reach, higher impact
Global reach, higher impact