13.3
Impact Factor
Theranostics 2021; 11(3):1207-1231. doi:10.7150/thno.48342 This issue Cite
Review
Treatment of severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease 2019 (COVID-19): a systematic review of in vitro, in vivo, and clinical trials
1. Department of Pediatrics, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Republic of Korea.
2. Department of Pediatrics, Yonsei University College of Medicine, Seoul, Republic of Korea.
3. Yonsei University College of Medicine, Seoul, Republic of Korea.
4. Department of Rheumatology, Yonsei University Wonju College of Medicine, Wonju, Republic of Korea.
5. Department of Nephrology, Yonsei University Wonju College of Medicine, Wonju, Republic of Korea.
6. Department of Psychiatry, Yonsei University Wonju College of Medicine, Wonju, Republic of Korea.
7. Research and development unit, Parc Sanitari Sant Joan de Déu/CIBERSAM, Universitat de Barcelona, Fundació Sant Joan de Déu, Sant Boi de Llobregat, Barcelona, Spain.
8. ICREA, Pg. Lluis Companys 23, 08010, Barcelona, Spain.
9. Department of Global Health and Population, Harvard T.H. Chan School of Public Health, 677 Huntington Avenue, Boston, USA.
10. Pain and Rehabilitation Centre, and Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden.
11. Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Mental Health Research Networking Center (CIBERSAM), Barcelona, Spain.
12. Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, UK.
13. Centre for Psychiatric Research, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden.
14. The Cambridge Centre for Sport and Exercise Sciences, Anglia Ruskin University, Cambridge, UK.
15. School of Social Work, University of Southern California, CA, USA.
16. Division of Urology, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
17. Department of Internal Medicine IV (Nephrology and Hypertension), Medical University Innsbruck, Innsbruck, Austria.
18. Department of Internal Medicine I (Gastroenterology, Hepatology, Endocrinology & Metabolism), Medical University Innsbruck, Innsbruck, Austria.
19. Department of Internal Medicine, St. Johann County Hospital, St. Johann in Tirol, Austria.
20. Department of Internal Medicine, University of Illinois College of Medicine at Peoria, Peoria, IL, USA.
21. Faculty of Medicine, University of Versailles Saint-Quentin-en-Yvelines, Montigny-le-Bretonneux, France.
Received 2020-5-18; Accepted 2020-10-22; Published 2021-1-1
Abstract
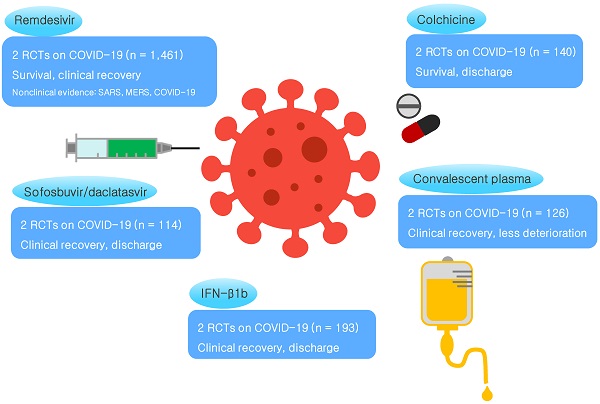
Rationale: Coronavirus disease 2019 (COVID-19) has spread worldwide and poses a threat to humanity. However, no specific therapy has been established for this disease yet. We conducted a systematic review to highlight therapeutic agents that might be effective in treating COVID-19.
Methods: We searched Medline, Medrxiv.org, and reference lists of relevant publications to identify articles of in vitro, in vivo, and clinical studies on treatments for severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and COVID-19 published in English until the last update on October 11, 2020.
Results: We included 36 studies on SARS, 30 studies on MERS, and 10 meta-analyses on SARS and MERS in this study. Through 12,200 title and 830 full-text screenings for COVID-19, eight in vitro studies, 46 randomized controlled trials (RCTs) on 6,886 patients, and 29 meta-analyses were obtained and investigated. There was no therapeutic agent that consistently resulted in positive outcomes across SARS, MERS, and COVID-19. Remdesivir showed a therapeutic effect for COVID-19 in two RCTs involving the largest number of total participants (n = 1,461). Other therapies that showed an effect in at least two RCTs for COVID-19 were sofosbuvir/daclatasvir (n = 114), colchicine (n = 140), IFN-β1b (n = 193), and convalescent plasma therapy (n = 126).
Conclusions: This review provides information to help establish treatment and research directions for COVID-19 based on currently available evidence. Further RCTs are required.
Keywords: COVID-19, therapeutic agent, SARS, MERS, mortality, coronavirus
Introduction
Coronavirus disease 2019 (COVID-19) refers to a respiratory syndrome caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an RNA virus belonging to the Coronaviridae family. Ever since the disease was first reported in Wuhan, China in December 2019, it has spread rapidly around the world. On October 28, 2020, a total of 43,766,712 SARS-CoV-2 cases were reported worldwide, of which 1,163,459 died [1]. Clinical manifestations range from being asymptomatic to pneumonia and acute respiratory distress syndrome (ARDS). Although estimations of case-fatality rate are different for COVID-19, there appears to be a high rate of a severe disease course or death, mainly in patients with advanced age or underlying diseases [2, 3]. Current case fatality rates are 2.2% in Africa, 3.2% in Americas, 2.5% in Eastern Mediterranean Region, 3.2% in Europe, 1.6% in South-East Asia, and 2.1% in Western Pacific Region [1], whereas the case fatality rate of SARS and Middle East respiratory syndrome (MERS), which are coronavirus respiratory syndromes similar to COVID-19 were 11% [4] and 34% [5], respectively.
There are currently no specific established treatments for COVID-19. Since the outbreak of COVID-19, numerous studies have been conducted during the past months; however, it is difficult to extract information from these extensive studies, synthesize the results, and apply them in practice. In fact, it would be almost impossible for front-line medical practitioners to be able to absorb the considerable number of reports being released on a daily basis and immediately translate the findings into practice during this medical crisis.
For this reason, we summarized the in vivo, in vitro, and clinical research results related to potential therapies of COVID-19 and further integrated the results with previously reported results from SARS and MERS. We aimed to provide useful information for the establishment of treatment and research directions for COVID-19.
Methods
Literature search strategy and study selection
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Two investigators (YJH and JIS) manually searched Medline for literature regarding therapeutics for SARS, MERS, and COVID-19. Only publications in English were included, with the exception of an individual study used within a meta-analysis.
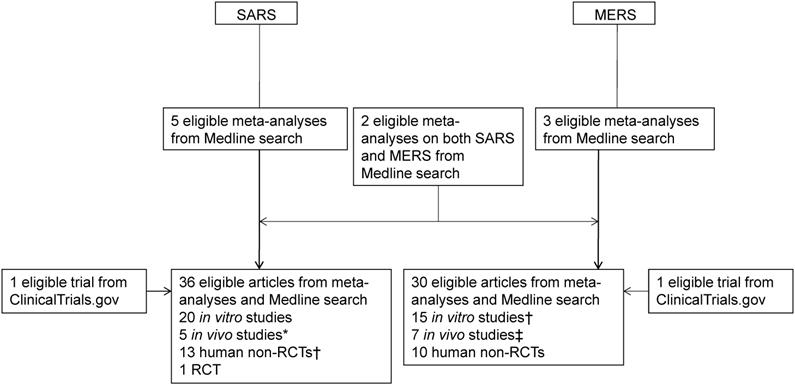
In order to complete this review in a timely manner during this pandemic, we first searched the meta-analyses or systematic reviews on SARS and MERS from inception to March 31, 2020 using the following search terms (“severe acute respiratory syndrome”, “SARS”, “Middle East respiratory syndrome”, or “MERS”) and (“meta”[title] or “systematic” [title]). After reading the full-text of articles obtained as a result of this search, we also investigated the in vitro, in vivo, and human studies on therapeutics of SARS or MERS that were included in them. Next, we conducted an additional search using the following search terms for the parts that were considered to be necessary for replenishment: [(“severe acute respiratory syndrome” or “SARS”) and (“remdesivir”, “nelfinavir”, “interferon beta”, or “chloroquine”)] or [(“Middle East respiratory syndrome” or “MERS”) and (“remdesivir”, “lopinavir”, “ritonavir”, “interferon alpha”, “interferon beta”, “convalescent plasma”, “chloroquine”, or “corticosteroid”)] (Figure 1).
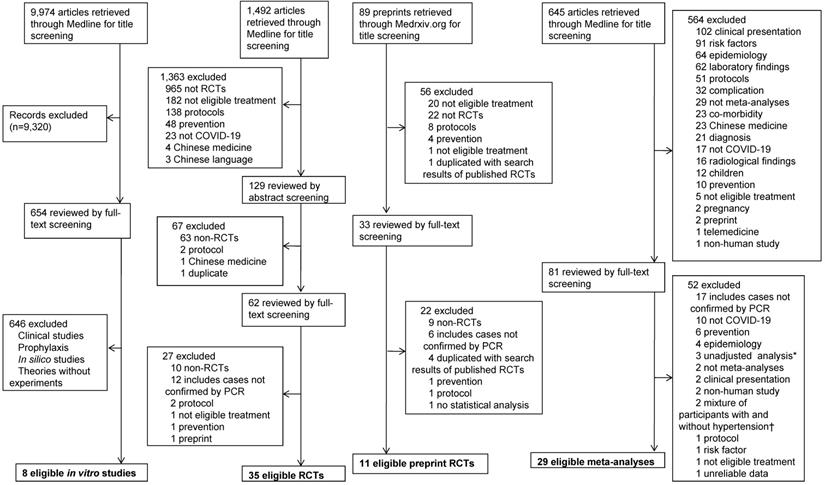
Moreover, in order to search for studies on COVID-19, a search was performed through the following search algorithm until the last update on May 7, 2020: ((wuhan[All Fields] and (“coronavirus”[MeSH Terms] or “coronavirus”[All Fields])) and 2019/12[PDAT]: 2030[PDAT]) or 2019-nCoV[All Fields] or 2019nCoV[All Fields] or COVID-19[All Fields] or SARS-CoV-2[All Fields]. In addition, a search for randomized controlled trials (RCTs) on COVID-19 was also performed using the following search terms until the last update on October 9, 2020: ((((wuhan[All Fields] and (“coronavirus”[MeSH Terms] or “coronavirus” [All Fields])) and 2019/12 [PDAT]: 2030[PDAT]) or 2019-nCoV[All Fields] or 2019nCoV [All Fields] or COVID-19[All Fields] or SARS-CoV-2[All Fields]) and (random [Title/Abstract] or randomization [Title/Abstract] or randomized [Title/Abstract] or randomized [Title/Abstract] or trial[Title]). To include a more sufficient amount of RCTs, a search for preprint RCTs through the database of Medrxiv.org was performed by conditions that include the following search terms in the titles until the last update on October 11, 2020: [“COVID” and (“random”, “controlled”, or “trial”)] or [“coronavirus” and (“random”, “controlled”, or “trial”)]or [“cov” and (“random”, “controlled”, or “trial”)]. A search for meta-analyses of treatment for COVID-19 was performed using the following search terms until the last update on October 11, 2020: ((((wuhan[All Fields] and (“coronavirus”[MeSH Terms] or “coronavirus”[All Fields])) and 2019/12[PDAT]: 2030[PDAT]) or 2019-nCoV[All Fields] or 2019nCoV[All Fields] or COVID-19[All Fields] or SARS-CoV-2[All Fields]) and (meta[Title]) (Figure 2).
Flowchart of article selection process for SARS and MERS. SARS: severe acute respiratory syndrome; MERS: Middle East respiratory syndrome; RCT: randomized controlled trial. *Two overlapped with in vitro studies on SARS; †One overlapped with an in vitro study on SARS each; ‡One overlapped with in vitro studies on MERS.
Flowchart of article selection process for COVID-19. COVID-19: coronavirus disease 2019; PCR: polymerase chain reaction; RCT: randomized controlled trial. *Including non-RCTs on corticosteroid therapy for patients with various severity of COVID-19. †Studies on angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
Eligibility criteria
Two investigators (YJH and JIS) identified the eligible studies by screening the titles and abstracts independently. Any disagreement was resolved by discussion and consensus among review authors. For non-human research, eligibility criteria for inclusion were (1) studies on SARS-CoV, MERS-CoV, or SARS-CoV-2 and (2) studies in which inoculation of virus preceded administration of therapeutic agents. For human research, eligibility criteria were organized in accordance with the Participants, Interventions, Comparisons, and Outcomes (PICO) reporting structure.
Participants
We included studies on individuals with SARS, MERS, or COVID-19 who were diagnosed by validated methods using real time reverse transcription polymerase chain reaction (PCR) [6]. We excluded studies that were performed exclusively in children. According to the 7th edition of the Chinese clinical guidance for COVID-19 pneumonia, treatment with corticosteroids, tocilizumab, or convalescent plasma was recommended for patients with severe or progressive COVID-19 [7]. Therefore, when a non-RCT was included in a meta-analysis and targeted any of these treatment forms for patients with different severity of COVID-19, only the study analyzing the results of multivariate analysis conducted in the original research was included. In the case of meta-analyses on angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) for COVID-19, studies that did not include participants selectively according to the presence of hypertension were excluded because it was thought that a mixture of participants with and without hypertension would affect the treatment outcome.
Interventions
We considered the pharmacological, immunological, or miscellaneous therapies administered after the onset of infection. Multiple therapeutic agents in combination were also included. Types of respiratory support, mechanical ventilation (MV) strategy, extracorporeal therapy, and radiation therapy were not target interventions in this study. The exclusion criteria were studies on (1) immunization or chemoprophylaxis, (2) Chinese medicine, or (3) other topics, such as epidemiology, without dealing with therapeutic interventions. We also excluded non-RCTs that did not specify the number of patients in the intervention group.
Comparisons
Control interventions relevant to the general treatment of respiratory infection (e.g., placebo or usual medications) or other therapeutic agents that could be candidates for the study intervention were included.
Outcomes
Studies reporting on mortality, intensive care unit (ICU) admission, disease progression, discharge rates, or improvement in the chest radiograph in the intervention/entire patient group or control group were included.
Study design
Because RCTs on SARS or MERS performed to date were not sufficient, any RCT, study in prospective or retrospective cohort design, case-control design, or case series published as an article in a scientific journal were eligible. In the case of non-RCTs, studies with a total of 10 or more patients were included, except for relatively rare treatment forms that had not been administered in dozens of patients to date. For COVID-19, only RCTs were eligible except of studies included in a meta-analysis.
Data extraction
Two investigators (YJH and JIS) collected information on the total number of patients and the number of patients in the intervention group, time range of enrollment or at the time of diagnosis or hospitalization, intervention and control therapy used in the study, and the outcome among the intervention and the control group.
Classification of studies and interpretation of results
In order to interpret the results of in vitro studies, 50% maximal effective concentration (EC50) less than 10 μM or selectivity index (SI) greater than 10 was set as a criterion for determining whether a particular drug has therapeutic potential against the virus of interest.
The results of RCTs and meta-analyses were categorized as follows, depending on whether the therapeutic agent was effective against COVID-19.
Effective: The treatment group showed superior results for major outcomes (mortality, ICU admission, disease progression, discharge, clinical improvement, or improvement in the chest radiograph) with a statistical significance (P < 0.05).
Possible effect: The major outcome of the treatment group was not significantly worse (P > 0.05), and the results for other outcomes other than the major outcome was superior in the treatment group with a statistical significance (P < 0.05).
Not effective: The results for any outcome did not show a significant difference between the treatment and the control group (P > 0.05).
Possible harm: The treatment group did not show statistically superior results for major outcomes (P > 0.05), and the results for other outcomes were worse with a statistical significance (P < 0.05).
Harmful: The treatment group showed statistically inferior results for the major outcome (P < 0.05).
Results
Systematic search results
Through Medline search, a total of 10 meta-analyses on SARS (n = 5) [8-12], MERS (n = 3) [13-15], and both (n = 2) [16, 17] were obtained and investigated. After investigating the original texts of in vitro, in vivo, and clinical studies cited in these meta-analyses, an additional Medline search was performed when the clinical study obtained by the search seemed to be insufficient for therapeutic agents that showed positive results from in vitro or in vivo studies. Through this process, 36 and 30 eligible articles on SARS and MERS were obtained, respectively: 20 in vitro, five in vivo studies (two overlapping with in vitro studies on SARS), 13 human non-RCTs (one overlapping with an in vitro study on SARS), and one RCT on SARS; and 15 in vitro (one overlapping with an in vitro study on SARS), seven in vivo studies (one overlapping with in vitro studies on MERS), and 10 human non-RCTs on MERS. In addition, as a result of searching the database of Clinicaltrials.gov, we identified one RCT on SARS that was not completed after registration, and one on MERS. Of these, an RCT of lopinavir/ritonavir plus ribavirin in the treatment of SARS [18] had not yet started recruiting participants since it was registered in December 2007, and the current status was unknown; and another RCT of lopinavir/ritonavir and interferon (IFN)-β1b in the treatment of MERS [19] was completed on May 20, 2020 (Figure 1).
A total of 12,200 articles on COVID-19 were identified through a Medline and Medrxiv.org search. After full-text screening of 830 articles, 83 eligible articles on COVID-19 were obtained: eight in vitro studies, 46 RCTs on 6,886 patients, and 29 meta-analyses (Figure 2).
The research results for SARS and MERS for each therapeutic agent are described in Table 1, and the research results for COVID-19 are described in Table 2, 3 & Table 4, and Table S1.
Antiviral agents
Remdesivir
Remdesivir showed effects in multiple non-human studies on SARS or MERS (Table 1), and in one in vitro study on COVID-19 (Table 2). Four RCTs on remdesivir for COVID-19 have been published to date. One of them was a large-scale RCT, with 538 and 521 participants in the treatment and the control group, respectively, and remdesivir was administered to the treatment group for 10 days. The time to recovery was shorter in the treatment group compared to the control group {11 [95% confidence interval (CI) 9-12] vs. 15 [13-19] days; relative risk (RR) for recovery 1.32, 95% CI 1.12 to 1.55; P < 0.0001} and the odds ratio (OR) for the improvement of the ordinal score on day 15 was 1.50 (95% CI 1.18 to 1.91, P = 0.001). There was no significant difference in the 14-day mortality rate between the two groups. However, when compared among the participants with a baseline ordinal score of 5 requiring oxygen supplementation, the 14-day mortality rate of the treatment group was significantly lower (4 out of 222 [2%] vs. 19 out of 199 [10%]; hazard ratio [HR] 0.22, 95% CI 0.08 to 0.58) [20]. In another RCT on severe COVID-19, the 28-day mortality rate did not differ between remdesivir-treated patients and controls [21] (Table 3). According to a meta-analysis for these two RCTs [20, 21], the RR for clinical recovery was 1.17 (95% CI 1.07 to 1.29) [22] (Table 4).
The other two RCTs for COVID-19 were performed with different administration periods of remdesivir, five and ten days, respectively. Among them, the 5-day treatment group showed better clinical status distribution on the 7-category ordinal scale on day 11 (OR 1.65, 95% CI 1.09 to 2.48) in one RCT for moderate COVID-19 [23]. In another RCT for severe COVID-19, the incidence of serious adverse events was lower in the 5-day treatment group than in the 10-day treatment group (42 out of 200 [21%] vs. 68 out of 197 [35%]; difference 10.8%, 95% CI 2.4% to 19.2%) [24] (Table 3). In a meta-analysis involving one of these RCTs [24] and another unreported RCT [25], the OR for clinical recovery in the 5-day course of treatment was 1.33 (95% CI 1.01 to 1.76) compared to the 10-day course of treatment [26] (Table 4).
Sofosbuvir and daclatasvir
A combination of sofosbuvir/daclatasvir showed an effect in two RCTs on COVID-19 which were conducted in Iran. In one RCT, the cumulative incidence of hospital discharge was higher (P = 0.041) and the duration of hospitalization was shorter (6 [interquartile range (IQR) 4-8] vs. 8 days [5-13]; P = 0.029) in the treatment compared to the control group [27]. In another RCT, the cumulative incidence of recovery was higher in the treatment compared to the control group (P = 0.033) [28] (Table 3).
Favipiravir
The results of two in vitro studies on favipiravir for COVID-19 were unfavorable [29, 30]. However, in a Russian RCT on favipiravir for moderate COVID-19, the rate of negative results of virus PCR on day 5 was higher in the treatment than in the control group (25 out of 40 [63%] vs. 6 out of 20 [30%]; P = 0.018) [31]. In another RCT on mild COVID-19, the hospital discharge rate of participants who received favipiravir from the first day of enrollment was higher than that of participants who received favipiravir starting from one week after enrollment (HR 2.68, 95% CI 1.67 to 4.29) [32] (Table 3).
Umifenovir
Umifenovir showed an effect in an in vitro study on COVID-19 [33] (Table 2). In an RCT comparing a combination of umifenovir and lopinavir/ritonavir with standard treatment, the group receiving the treatment with umifenovir did not show better outcome than the control group in terms of clinical deterioration or viral clearance [34] (Table 3). On the other hand, a meta-analysis that included this RCT [34] and four observational studies on COVID-19 demonstrated that umifenovir treatment enhanced the rate of viral clearance on day 14 (RR 1.27, 95% CI 1.04 to 1.55; P = 0.02; I2 = 63%; n = 683) [35] (Table 4).
Summary of studies evaluating therapeutics for SARS and MERS
| Therapeutics | SARS | MERS | ||||
|---|---|---|---|---|---|---|
| In vitro | In vivo | Human | In vitro | In vivo | Human | |
| Antiviral agents | ||||||
| Ribavirin | 4 studies [36, 130-132] 2 studies [133, 134] | 4 studies [135-138] | 1 study [139] | |||
| Remdesivir | 1 study [140] | 1 study [140] | 4 studies [38, 140-142] | 2 studies [38, 143] | ||
| Lopinavir | 1 study [130] 2 studies [48, 50] | 2 studies [38, 51] 1 study [95] | ||||
| Ritonavir | 1 study [48] | |||||
| Oseltamivir | 1 study [136] | 1 study [144] | ||||
| Nelfinavir | 1 study [48] 1 study [50] | 1 study [50] | ||||
| Interferon | IFN-α (8 studies) [50, 130, 132, 134, 145-148]; IFN-β (8 studies) [130, 132, 145, 147-151] | IFN-α/IL-1β (1 study) [152] IFN-α B/D, rintatolimod† (1 study) [50]. | IFN-α (1 study): more effective than corticosteroids [153]. | IFN-α (1 study) [139]; IFN-β (2 studies) [154, 155]. | IFN-β (2 studies) [39, 156]§ | 1 study [14]‡ |
| IFN-α-n3 (1 study) [50] | ||||||
| Combination therapy based on antiviral agents or interferon | ||||||
| Ribavirin/IFN | IFN-α (1 study) [130]; IFN-β (2 studies) [130, 131]. | IFN-α (1 study) [157] | IFN-α (2 studies) CFR 6/20 (30%) vs. 17/24 (71%) (P = 0.01) [158] CFR 14/61 (23%) vs. 2/2 (100%) (P = 0.01) [159]. | |||
| 4 studies [144, 160-162]: no difference in mortality. | ||||||
| Ribavirin/lopinavir | 1 study [36] | |||||
| Ribavirin plus L/r | Registered RCT(not yet recruiting) [18] | |||||
| L/r | 3 studies [36, 99, 130] 1 study [48] | 2 studies: Rates of ARDS/death (2% vs. 29%, P = 0.001) [36] CFR 2% vs. 16% (P < 0.05) [37]. | 1 study [38] | 1 study [39] | ||
| L/r plus IFN-β | 2 studies [38, 39] | Ongoing RCT [19] | ||||
| Ribavirin/corticosteroids | 1 study earlier administration [163] | |||||
| IFN-α/corticosteroids | 1 study [153] | |||||
| IFN-β/IFN-γ | 1 study [164] | |||||
| Intranasal IFN-β/ HR2P-M2 | 1 study [156] | |||||
| Antibiotics | ||||||
| Macrolide | 1 study: mortality and viral clearance [165] | |||||
| 4-Aminoquinoline | ||||||
| Chloroquine | 3 studies [50, 166, 167] | 1 study [50] | 1 study [51] 1 study [52] | |||
| Amodiaquine | 1 study [50] | 1 study [50] | ||||
| Corticosteroids | 4 studies Higher dose [62, 63] High dose methylprednisolone [64] Methylprednisolone was better than 3 other groups (no steroid, hydrocortisone, or pulse therapy) [65]. | Inconclusive (2 studies); Delay in viral clearance (HR 0.35; 95% CI 0.17-0.72), not associated with mortality [61] CFR 6/13 (46%) vs. 2/19 (11%) (P = 0.04) in univariate analysis [144]. | ||||
| Inconclusive (2 studies) [59, 60] Early administration - higher plasma viral load, no difference in severity [59]* Possible adverse effect (1 study): osteonecrosis [10]‡. | ||||||
| Immunotherapy | ||||||
| Convalescent plasma | Inconclusive (1 study) CFR [0/1 (0%) vs. 2/28 (7%)] and [0/19 (0%) vs. 5/21 (24%) in 2 comparative studies, and 0/1, 0/1, 0/3, and 10/80 in 4 non-camparative studies [12]‡ | 1 study [168] | Inconclusive (1 study): 2 out of 3 cases with respiratory failure showed neutralizing activity [169]. | |||
| Monoclonal antibody | 201 [170] | m336 [168], hMS-1 [171], 4C2h [172], HR2P-M2 [156]. | ||||
| Other drugs | β-D-N4-hydroxycytidine [50]; calpain inhibitor VI [50] | Camostat [173] | Camostat [80], loperamide [51], chlorpromazine [51, 52, 174], imatinib [174, 175], saracatinib [80, 175], baricitinib [80], dasatinib [174], cyclosporine [176], EST [80], cathepsin L/K inhibitor [80], gemcitabine/toremifene/ triflupromazine [174], mycophenolic acid [155]. | |||
| β-D-N4-hydroxycytidine [50]; calpain inhibitor VI [50] | Toremifene [52] | |||||
(Effective; bold Not effective). In the outcome description, the former is the data of the treatment group and the latter is the data of the control group.
CFR: case-fatality ratio; CI: confidence interval; EST: (23,25)-trans-epoxysuccinyl-l-leucylamindo-3-methylbutane ethyl ester; HR: hazard ratio; ICU: intensive care unit; IFN: interferon; L/r: lopinavir/ritonavir; MERS: Middle East respiratory syndrome; OR: odds ratio; RCT: randomized controlled trial; RR: risk ratio; SARS: severe acute respiratory syndrome;
*This study is the only published randomized controlled trial in this table. †A mismatched double-stranded RNA interferon inducer. ‡Meta-analysis. §Intranasal administration.
Therapeutic agents that showed effects against SARS-CoV-2 in in vitro studies
| Therapeutics | First author | Findings | Conclusion |
|---|---|---|---|
| Antiviral agents | |||
| Umifenovir | Wang [33] | EC50 = 4.11 μM; CC50 = 31.79 μM; SI = 7.73 | Potent |
| Remdesivir | Wang [29] | EC50 = 0.77 μM; CC50 > 100 μM; SI > 129.87 | Potent |
| Choy [30] | EC50 = 26.9 μM; CC50 > 100 μM | Not potent | |
| Nelfinavir | Musarrat [49] | Complete inhibition of SARS CoV-2 mediated cell fusion at 10 μM | Potent |
| Antiparasitic agents | |||
| Ivermectin | Caly [177] | 5000-fold reduction in viral RNA at 48h after a single administration (IC50 < 2mM) | Potent |
| Emetine | Choy [30] | EC50 = 0.5 μM; CC50 = 56.46 μM | Potent |
| 4-aminoquinoline (anti-malarial agents) | |||
| Chloroquine | Wang [29] | EC50 = 1.13 μM; CC50 > 100 μM; SI > 88.5 | Potent |
| Yao [53] | Incubation time may influence antiviral activity (24h EC50 = 23.9 μM; 48h EC50 = 5.47 μM). | Potent | |
| Liu [54] | EC50 = 2.71 (MOI = 0.01), 3.81 (0.02), 7.14 (0.2), 7.36 (0.8) μM; CC50 = 273.2 μM | Potent | |
| Hydroxychloroquine | Yao [53] | 24h EC50 = 6.14 μM; 48h EC50 = 0.72 μM | Potent |
| Liu [54] | EC50 = 4.51 (MOI = 0.01), 4.06 (0.02), 17.31 (0.2), 12.96 (0.8) μM; CC50 = 249.5 μM | Potent | |
| Other agents | |||
| Homoharrngtonine | Choy [30] | EC50 = 2.14 μM; CC50 = 59.75 | Potent |
| Nitazoxanide | Wang [29] | EC50 = 2.12 μM; CC50 > 35.53 μM; SI > 16.76 | Potent |
| Immunotherapy | |||
| EK1C4 | Xia [178] | IC50 = 36.5 nM; CC50 > 5 μM; SI > 136 | Potent |
CC50: 50% cytotoxic concentration; COVID-19: coronavirus disease 2019; EC50: 50% maximal effective concentration; MOI: multiplicity of infection; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SI: selectivity index.
Summary of RCTs evaluating therapeutics for COVID-19
| Therapeutics (daily dosage mg) [Common treatment applied to all participants] | First author | Condition | Region | Period of enrollment | No. of participants (treatment - control group) | Outcome of patients or findings: treatment group vs. control group (number of participants or the median value [IQR]) | Conclusion |
|---|---|---|---|---|---|---|---|
| Antiviral agents | |||||||
| Remdesivir | |||||||
| 200 mg (day 1); 100 mg (day 2-10) vs. standard treatment | Beigel [20] | Not specified | World-wide* | Feb 21-Apr 19 | 541 - 521 | Improvement in the ordinal score on day 15: OR 1.50, 95% CI 1.18 to 1.91 (P = 0.001) 14-day mortality: 32 (6%) vs. 54 (10%) (HR 0.70, 95% CI 0.47 to 1.04) 14-day mortality in patients with a baseline ordinal score of 5 (requiring oxygen): 4/222 (2%) vs. 19/199 (10%) (HR 0.22, 95% CI 0.08 to 0.58) Time to recovery: 11 (95% CI 9-12) vs. 15 (13-19) days (RR for recovery 1.32, 95% CI 1.12 to 1.55; P < 0.0001). | Effective |
| A: 200 mg (day 1); 100 mg (day 2-10) B: 200 mg (day 1); 100 mg (day 2-5). C: Standard treatment. | Spinner [23] | Moderate | The US, Europe, Asia | Mar 15-Apr 18 | 197 (A) 199 (B) 200 (C) | Better clinical status distribution on the 7-category ordinal scale on day 11: OR (B vs. C) 1.65 (95% CI 1.09 to 2.48) 28-day mortality: 3 (A, 2%); 2 (B, 1%); 4 (C, 2%). | Effective (5-day treatment) |
| 200 mg (day 1); 100 (day 2-10) vs. standard treatment | Wang [21] | Severe | China | Feb 6-Mar 12 | 158 - 78 | 28-day mortality: 22 (14%) vs. 10 (13%) Time to clinical improvement (a 2-point reduction on a 6-category ordinal scale, or discharge from hospital): 21 [13-28] vs. 23 [15-28] days (HR 1.23, 95% CI 0.87 to 1.75) | Not effective |
| A: 200 mg (day 1); 100 (day 2-5) B: 200 mg (day1); 100 (day 2-10) | Goldman [24] | Severe | World-wide† | Mar 6-Mar 26 | 200 (A) 197 (B) | Clinical improvement of 2 points or more on a 7-category ordinal scale within 14 days: 129 (A, 64%) vs. 107 (B, 54%) (difference -6.5%, 95% CI -15.7% to 2.8%) 14-day mortality among patients receiving MV or ECMO: 10/25 (A, 40%) vs. 7/41 (B, 17%) Serious adverse event: 42 (A, 21%) vs. 68 (B, 35%) (difference 10.8%, 95% CI 2.4% to 19.2%). | Favors 5-day treatment |
| Sofosbuvir/daclatasvir | |||||||
| 400 mg/60 mg for 14 days vs. standard treatment [hydroxychloroquine with or without lopinavir /ritonavir] | Sadeghi [27] | Moderate/severe | Iran | Mar 26-Apr 26 | 33 - 33 | Duration of hospitalization: 6 [4-8] vs. 8 days [5-13] (P = 0.029) The cumulative incidence of hospital discharge was higher in the treatment group (P = 0.041). Clinical recovery within 14 days: 29 (88%) vs. 22 (67%) (P = 0.076). | Effective |
| 400 mg/60 mg plus ribavirin (1,200) vs. hydroxychloroquine and lopinavir/ritonavir with/without ribavirin | Abbaspour Kasgari [28] | Moderate | Iran | Mar 20-Apr 8 | 24 - 24 | ICU admission: 0 (0%) vs. 4 (17%) (P = 0.109) Hospital mortality 0 (0%) vs. 3 (13%) (P = 0.234). The cumulative incidence of recovery was higher in the treatment group (P = 0.033). | Effective |
| Favipiravir | |||||||
| A: 3,200 mg (day 1); 1,200 (day 2-14); B: 3,600 day (day 1); 1,600 (day 2-14); C: Standard treatment. | Ivashchenko [31] | Moderate | Russia | Apr-May | 20 (A) 20 (B) 20 (C) | Discharge or achievement of score 2 on WHO-OSCI by day 15: 13 (A, 65%), 17 (B, 85%), and 17 (C, 85%) Viral clearance on day 5: 25/40 (A and B, 63%) vs. 6/20 (C, 30%) (P = 0.018) | Possible effect |
| 3,600 mg (day 1); 1,600 mg (day 2-10) vs. 3,600 mg (day 6); 1,600 mg (day 7-15) | Doi [32] | Mild | Japan | Mar 2-May18 | 36 - 33 | Time to discharge from the hospital: 14.0 vs.21.5 days (HR 2.68, 95% CI 1.67 to 4.29) Viral clearance on day 6: 67% vs. 56% (adjusted HR 1.42, 95% CI 0.76 to 2.62) 69 out of 82 participants (84%) developed hyperuricemia. | Favors early treatment |
| Other antiviral agents | |||||||
| Lopinavir (800)/ ritonavir (200) for 14 days vs. standard treatment | Cao [40] | Severe | China | Jan 18-Feb 3 | 99 - 100 | 28-day mortality: 19 (19%) vs. 25 (25%) (difference -5.8%; 95% CI -17.3% to 5.7%) Time to clinical improvement (a 2-point reduction on a 7-category ordinal scale or discharge from hospital): 16 [13-17] vs. 16 [15-18] days (HR 1.24, 95% CI 0.90 to 1.72) Hospital stay: 14 [12-17] vs. 16 [13-18] days (difference 1, 95% CI 0 to 2) | Not effective |
| A: Lopinavir (400) /ritonavir (100) for 7-14 days B: Umifenovir (600) for 7-14 days C: Standard treatment | Li [34] | Mild/ moderate | China | Feb 1-Mar 28 | 34 (A) 35 (B) 17 (C) | Deterioration to severe/critical COVID-19 on day 7: 8/34 (A, 24%), 3/35 (B, 9%), and 2/17 (C, 12%) (P = 0.206) Time to viral clearance: 9.0 (A; SD 5.0), 9.1 (B; 4.4), and 9.3 (C; 5.2) days (P = 0.981) Viral clearance within 7 days: 12/34 (A, 35%), 13/35 (B, 37%), and 7/17 (C, 41% ) (P = 0.966). | Not effective |
| A: Ribavirin (2,000 mg loading; 1,200-1,800 mg for 14 days) B: Lopinavir (800)/ritonavir (200) C: Ribavirin plus lopinavir/ritonavir | Huang [41] | Mild/ moderate | China | Jan 29-Feb 25 | 33 (A) 36 (B) 32 (C) | Deterioration to severe COVID-19: 1 (A, 3%), 2 (B, 6%), and 2 (C, 6%) (P = 0.58) Time to viral clearance: 13.0 [9.0-25.5] (A), 12.0 [7.0-19.0] (B), and 15.0 [9.3-17.8] (C) days (P = 0.42) Viral clearance on day 14: 17/33 (A, 52%), 22/36 (B, 61%), and 15/32 (C, 47%). | Not effective |
| Azvudine (FNC) (5) vs. standard treatment | Ren [45] | Mild/ moderate | China | Feb 18-Feb 29 | 10 - 10 | Time to radiological improvement was shorter in the treatment group (P = 0.0401). Viral clearance on day 6: 10 (100%) vs. 4 (40%) (P = 0.0011). | Effective |
| Triazavirin (750 or 1,000 for 7 days)‡ vs. standard treatment | Wu [46] | Not specified | China | Feb 14-Mar 6 | 26 - 26 | Clinical improvement§: 10 (39%) vs. 6 (23%) (RR 2.1, 95% CI 0.6 to 7.0, P = 0.2) Time to clinical improvement§: 7 [6-15] vs. 12 [7-16] days (RR 2.0, 95% CI 0.7 to 5.6, P = 0.2). | Not effective |
| Darunavir (800)/cobicistat (150) for 5 days vs. standard treatment | Chen [47] | Mild | China | Jan 30-Feb 6 | 15 - 15 | Worsening of chest CT findings: 7 (47%) vs. 4 (27%) (P = 0.45) Viral clearance on day 7: 7 (47%) vs. 9 (60%) (P = 0.72). | Not effective |
| Hydroxychloroquine | |||||||
| 800 mg (day 1); 400 mg (day 2-7) vs. standard treatment | Mitjà [179] | Mild | Spain | Mar 17-Apr 28 | 136 - 157 | Number of hospitalized participants: 8 (6%) vs. 11 (7%) (RR 0.75, 95% CI 0.32 to 1.77); Time to the resolution of symptoms : 10 [4-18] vs. 12 [6-21] days (P = 0.38); Reduction in viral load on day 7: -3.49 (SD 0.20) vs. -3.37 (0.19) log10 copies/mL (difference -0.12, 95% CI -0.25 to 0.5). | Not effective |
| 1,200 mg (day 1-3); 800 mg (day 4-14) vs. standard treatment | Tang [180] | Mild/ moderate | China | Feb 11-Feb 29 | 75 - 75 | Alleviation of symptoms by day 28¶: 60% vs. 67% (difference -7%, 95% CI -41% to 28%) Viral clearance by day 28: 56 (75%) vs. 53 (71%) Adverse events**: 21/70 (30%) vs. 7/80 (9%). | Not effective |
| 800 mg (day 1); 400mg (day 2-15) vs. standard treatment | Abd-Elsalamb [181] | Not specified | Egypt | Mar-Jun | 97 - 97 | Initiation of MV: 4 (4%) vs. 5 (5%) (P = 0.75) 28-day mortality: 6 (6%) vs. 5 (5%) (P = 0.77). | Not effective |
| 1,400 mg (day 1); 600 mg (day 2-5) vs. standard treatment | Skipper [182] | Mild | The US, Canada | Mar 22-May 20 | 73 - 72 | Change in symptom severity score over 14 days: -2.21(SE 0.23) vs. -2.10 (0.23) (P = 0.51). | Not effective |
| 800 mg (day 1); 400 mg (day 2-5) vs. standard treatment | Kamran [56]†† | Mild | Pakistan | Apr 10-May 31 | 349 - 151 | Disease progression‡‡: 11 (3%) vs. 5 (3%) (P = 0.865) Viral clearance within 7 days: 182 (52%) vs. 54 (36%) (P = 0.001). | Possible effect |
| 400 mg for 5 days vs. standard treatment | Chen [55]†† | Mild/ moderate | China | Feb 4-Feb 28 | 31 - 31 | Improvement of chest CT scans on day 6: 25 (81%) vs. 17 (55%) (P = 0.0476) Duration of fever: 2.2 (SD 0.4) vs. 3.2 (1.3) days (P = 0.0008). | Effective |
| 800 mg (day 1); 400 mg (day 2-7) vs. standard treatment | Chen [183]†† | Mild/ moderate | Taiwan | Apr 1-May 31 | 21 - 12 | Clinical recovery (3 consecutive negative results of viral PCR and resolution of major symptoms) within 14 days: 6/21 (29%) vs. 5/12 (42%) (P = 0.51) Time to viral clearance: 5 (95% CI 1 to 9) vs. 10 (2 to 12) days (P = 0.40) Viral clearance within 14 days: 17/21 (81%) vs. 9/12 (75%) (P = 0.36). | Not effective |
| Azithromycin | |||||||
| 500 mg for 10 days vs. standard treatment | Furtado [58] | Severe | Brazil | Mar 28-May 19 | 214 - 183 | Worse clinical status on the 6-category ordinal scale on day 15: OR 1.36 (95% CI 0.94 to 1.97, P = 0.11) 28-day mortality: 90 (42%) vs. 73 (40%) (HR 1.08, 95% CI 0.79 to 1.47, P = 0.63). | Not effective |
| 500 mg for 5 days vs. standard treatment [Lopinavir/ritonavir and hydroxychloroquine] | Sekhavati [57] | Not specified | Iran | Apr 24-May 8 | 56 - 55 | Length of hospital stay: 4.6 (SD 2.6) vs. 6.0 (SD 3.2) days (P = 0.02) Mortality: 0 (0%) vs. 1 (2%) (P = 0.495) ICU admission: 2 (4%) vs. 7 (13%) (P = 0.070). | Effective |
| Colchicine | |||||||
| 2 mg (day 1)§§; 1 mg (till discharge or day 21) vs. standard treatment [Chloroquine or hydroxychloroquine and azithromycin]¶¶ | Deftereos [70] | Not specified | Greece | Apr 3-Apr 27 | 55 - 50 | Cumulative event-free 10-day survival rate: 97% vs. 83% (P = 0.03) Deterioration by 2 points on a 7-category ordinal scale within 3 weeks: 1 (2%) vs. 7 (14%) (OR 0.11, 95% CI 0.01 to 0.96, P = 0.046) Peak D-dimer concentration: 0.76 [0.41-1.59] vs. 0.92 [0.68-2.77] μg/mL (P = 0.04). | Effective |
| 1.5 mg (day 1-5); 1 mg (day 6-10) vs. standard treatment [azithromycin, hydroxychloroquine, and unfractionated heparin]. | Lopes [71]†† | Moderate/severe | Brazil | Apr 11-Jul 06 | 17 - 18 | Proportion of participants requiring supplemental oxygen on day 7: 6% vs. 39% (P = 0.01) Maintenance of hospitalization: 53% vs. 78% (on day 5), 6% vs. 17% (on day 10) (P = 0.01) Duration of oxygen supplement: 3.0 [1.5-6.5] vs. 7.0 [3.0-8.5] days (P = 0.02) Length of hospital stay: 6.0 [4.0-8.5] vs. 8.5 [5.5-11.0] days (P = 0.03). | Effective |
| Other agents | |||||||
| Methylprednisolone (250 for 3 days) vs.standard treatment [Hydroxychloroquine, lopinavir, naproxen]. | Edalatifard [66] | Severe | Iran | Apr 20-Jun 20 | 34 - 28 | Mortality: 2 (6%) vs. 12 (43%) (P < 0.001) Clinical improvement***: 32 (94%) vs. 16 (57%) (P = 0.001) Time to clinical improvement***: 11.8 (SD 4.9) vs. 16.4 (SD 6.9) (P = 0.003). | Effective |
| Telmisartan (160) for 14 days vs. standard treatment | Duarte [72]†† | Not specified | Argentina | May 14-Jul 30 | 41 - 41 | HR for discharge: 2.02 (95% CI 1.14 to 3.59) Time to discharge from the hospital: 9 vs. 15 days (P = 0.0124) 30-day mortality: 2/38 (5%) vs. 4/34 (12%) (P = 0.41) Serum CRP levels on day 5: 24.2 (SD 31.4) vs. 51.1 (44.8) mg/L (P < 0.05). | Effective |
| Enoxaparin (0.75-2 mg/kg for 4-14 days) vs. enoxaparin (40 or 80) or unfractionated heparin (15,000-22,500 IU)††† | Lemos [75] | Severe and intubated | Brazil | Apr-Jul | 10 - 10 | Successful liberation from MV by day 28: 8 (80%) vs. 3 (30%) (HR 4.0, 95% CI 1.04 to 15.05, P = 0.031) Ventilator-free days: 15 [6-16] vs. 0 [0-11] days (P = 0.028)]; 28-day mortality: 1 (10%) vs. 3 (30%) (P = 0.264). | Effective |
| Calcifediol (0.532 on day 1; 0.266 on day 3 and 7, then weekly) vs. standard treatment [hydroxychloroquine, azithromycin] | Entrenas Castillo [78] | Not specified | Spain | Not specified | 50 - 26 | ICU admission: 1 (2%) vs. 13 (50%) (adjusted OR 0.03, 95% CI 0.003 to 0.25). | Effective |
| CM4620-IE (Auxora: calcium release-activated calcium channel inhibitor) (2.0 mg/kg/day continuous infusion on day 1; 1.6 mg/kg/day on day 2-3) vs. standard treatment. | Miller [79] | Severe/ critical | The US | Apr 8-May 13 | 20 - 10 | IMV or death by day 30: 3/17 (18%) vs. 5/9 (56%) in participants with severe COVID-19 (HR 0.23, 95% CI 0.05 to 0.96; P < 0.05) The mean difference in 8-point ordinal scale between groups was statistically significant at day 6 and day 9-12 (P < 0.05). | Effective |
| Ruxolitinib (Janus-associated kinase inhibitors) (10) vs. standard treatment | Cao [81] | Severe | China | Feb 9-Feb 28 | 20 - 21 | Improvement of chest CT scans on day 14: 18 (90%) vs. 13 (62%) (P = 0.0495) 28-day mortality: 0 (0%) vs. 3 (14%) (P = 0.232) Time to clinical improvement (a 2-point reduction on a 7-category ordinal scale or discharge from hospital): 12 [10-19] vs. 15 [10-18] days (P = 0.147) (HR 1.669, 95% CI 0.836 to 3.335) Time to lymphocyte recovery: 5 [2-7] vs. 8 [2-11] days (P = 0.033). | Effective |
| Leflunomide (DHODH inhibitor) (100 day 1-3; 20 day 4-10) vs. standard treatment [Umifenovir]. | Hu [82] | Moderate | China | Feb 20-Feb 28 | 5 -5 | Duration of viral shedding: 5 vs. 11 days (P = 0.046) The difference in the level of serum CRP measured before treatment and on day: 32 [5.6-not tested] vs.0 [0-9.1] mg/L (P = 0.047). | Possible effect |
| Immunotherapy | |||||||
| Interferon | |||||||
| IFN-β1a (12 million IU 3 times weekly for 2 weeks) vs. standard treatment [Hydroxychloroquine plus lopinavir/ritonavir or atazanavir/ritonavir]. | Davoudi-Monfared [83] | Severe | Iran | Feb 29-Apr 3 | 42 - 39 | 28-day mortality: 19% vs. 44% (P = 0.015) Rate of discharge from the hospital: 67% vs. 44% (OR 2.5, 95% CI 1.05 to 6.37) Early administration of IFN-β1a reduced mortality (OR 13.5, 95% CI 1.5 to 118). Time to clinical improvement: 9.7 ± 5.8 vs. 8.3± 4.9 days (P = 0.95). | Effective |
| IFN-β1b (3 doses of 8 million IU on alternate days) plus ribavirin (800) for 14 days vs. standard treatment [Lopinavir/ritonavir] | Hung [44] | Mild/ moderate | Hong Kong | Feb 10-Mar 20 | 86 - 41 | Time to a NEWS2 of 0: 4 [3-8] vs. 8 [7-9] days (HR 3.92, 95% CI 1.66 to 9.23) Time to a SOFA score of 0: 3.0 [1.0-8.0] vs. 8.0 [6.5-9.0] days (HR 1.89, 95% CI 1.03 to 3.49) Length of hospital stay: 9.0 [7.0-13.0] vs. 14.5 [9.3-16.0] days (HR 2.72, 95% CI 1.2 to 6.13) Time to viral clearance: 7 [5-11] vs. 12 [8-15] days (HR 4.37, 95% CI 1.86 to 10.24, P = 0.001). | Effective |
| IFN-β1b (250 mcg on alternate days for 2 weeks) vs. standard treatment | Rahmani [84] | Severe | Iran | Apr 20-May 20 | 33- 33 | Discharge from hospital by day 14: 26 (79%) vs. 18 (55%) (OR 3.09, 95% CI 1.05 to 9.11, P = 0.03) ICU admission: 14 (42%) vs. 22 (67%) (P = 0.04) Time to clinical improvement (a 2-point reduction on a 6-category ordinal scale): 9 [6-10] vs. 11 [9-15] days (P = 0.002). | Effective |
| Inhaled IFN-κ (2) plus TFF2 (5) for 6 days vs. standard treatment | Fu [85] | Moderate | China | Mar 23-May 23 | 40 - 40 | Time to improvement of chest CT: 6.2 (95% CI 5.1-7.3) vs. 8.8 (95% CI 7.6-10.0) days (P = 0.002) Time to viral clearance: 3.8 (95% CI 2.1-5.5) vs. 7.4 (95% CI 4.6-10.2) days (P = 0.031). | Effective |
| A: Novaferon (40 mcg) B: Novaferon and lopinavir (800)/ritonavir (200) C: Lopinavir/ritonavir. | Zheng [86] | Moderate/ severe | China | Feb 1-Feb 20 | 30 (A) 30 (B) 29 (C) | Viral clearance on day 6: 15/30 (A, 50%; P = 0.04) or 18/30 (B, 60%; P = 0.0053) vs. 7/29 (C, 24%) Time to viral clearance: 6 (A, P = 0.417) or 6 (B, P = 0.036) vs. 9 (C) days. | Possible effect |
| Convalescent plasma | |||||||
| 4-13 mL/kg vs. standard treatment | Li [87] | Severe/ life-threatening | China | Feb 14-Apr 1 | 52 - 51 | Clinical improvement (a 2-point reduction on a 6-category ordinal scale or discharge from hospital) within 28 days: 27 (52%) vs. 22 (43%) (HR 1.40, 95% CI 0.79 to 2.49) Clinical improvement within 28 days for the participants with severe COVID-19: 21/23 (91%) vs. 15/22 (28%) (HR 2.15, 95% CI 1.07 to 4.32) 28-day mortality: 8 (16%) vs. 12 (24%) (OR 0.59, 0.22 to 1.59) Viral clearance within 72 hours: 41 (87%) vs. 15 (38%) (OR 11.39, 95% CI 3.91 to 33.18). | Effective (severe COVID-19 subgroup) |
| 200 mL (day1-2) vs. standard treatment | Agarwal [89]†† | Moderate | India | Apr 22-Jul 14 | 235 - 229 | 28-day mortality: 34 (15%) vs. 31 (14%) (adjusted OR 1.06, 95% CI 0.61 to 1.83) Disease progression (PaO2/FiO2 < 100): 44 (19%) vs. 41 (18%) (adjusted OR 1.09, 95% CI 0.67 to 1.77). | Not effective |
| 300 mL vs. standard treatment | Gharbharan [184]†† | Not specified | Nether-lands | Apr 8-Jun 10 | 43 - 43 | 60-day mortality: 6 (14%) vs. 11 (26%) (OR 0.95, 95% CI 0.20 to 4.67) Improvement in WHO-OSCI on day 15: 25 (58%) vs. 25 (58%) (OR 1.30, 95% CI 0.52 to 3.32). | Not effective |
| 250-300 mL vs. standard treatment | Avendaño-Solà [88]†† | Not specified | Spain | Apr 4-Jul 10 | 38 - 43 | Initiation of MV or death by day 15: 0 (0%) vs. 6 (14%) (P = 0.03) 28-day mortality: 0 (0%) vs. 4 (9%) (P = 0.06). | Effective |
| 200 mL (day1-2) vs. deferred treatment‡‡‡ | Barcells [185]†† | At risk for progression | Chile | May 10-Jul 18 | 28 - 30 | A composite of MV, hospitalization for > 14 days, or death: 9 (32%) vs. 10 (33%) (OR 0.95, 95% CI 0.32 to 2.84) 13 participants (43%) from the deferred group received convalescent plasma based on clinical aggravation. | Not effective |
| Other immunotherapies | |||||||
| rhG-CSF 5 mcg/kg (day 1-3) vs. standard treatment | Cheng [90] | Lympho-penia | China | Feb 18-Apr 10 | 100 - 100 | 21-day mortality: 2 (2%) vs. 10 (10%) (HR 0.19, 95% CI 0.04 to 0.88) Disease progression§§§: 2 (2%) vs. 15 (15%) (difference -13%, 95% CI -21.4% to -5.4%) Time to clinical improvement (a 1-point reduction on a 7-category ordinal scale or discharge from hospital): 12 [10-16] vs. 13 [11-17] (HR 1.28, 95% CI 0. 95-1.71, P = 0.06). | Effective (lympho-penia) |
| Intravenous immunoglobulin 0.5g/kg/day for 3 days plus methylprednisolone (40 mg once) vs. standard treatment | Sakoulas [91]†† | Moderate/severe (except patients with MV) | The US | May 1-Jun 16 | 16 - 17 | (Among subjects with alveolar-arterial oxygen gradient of >200 mmHg at enrollment) Initiation of MV within 30 days: 2/14 (14%) vs. 7/12 (58%) (P = 0.038), Length of hospital stay: 11 (range 5-22) vs. 19 (4-30) days (P = 0.013) Length of ICU stay: 2.5 (range 0-16) vs. 12.5 (1-29) days (P = 0.006) Difference in PaO2/FiO2 on day 7: +131 (+35 to +330) vs.+44.5 (-115 to +157) (P = 0.01). | Effective |
| Vilobelimab (anti-C5a antibody IFX-1) 800 mg (day 1, 2, 4, 8, and 15) vs. standard treatment | Vlaar [92] | Severe | Nether-lands | Mar 31-Apr24 | 15 - 15 | 28-day mortality: 2 (13%) vs. 4 (27%) (adjusted HR 0.65, 95% CI 0.10 to 4.14) Difference in the change in PaO2/FiO2on day 5 (least squares mean): 17% (SD 63) vs. 41% (difference -24%, 95% CI -58% to 9%, P = 0.15). | Not effective |
| CIGB-325 (anti-CK2) 2.5 mg/kg (day 1-5) vs. standard treatment | Cruz [93]†† | Not specified | Cuba | Jun 1-Jun 16 | 10 - 10 | Reduction in the number of pulmonary lesions on the chest CT: 5/6 (83%) vs. 3/7 (43%) (Bayesian P (difference > 0) = 0.951). Time to viral clearance: 11 (SD 8) vs. 12 (SD 6) days (P = 0.614). | Effective |
CI: confidence interval; COVID-19: coronavirus disease 2019; CRP: C-reactive protein; CT: computed tomography; DHODH: dihydroorotate dehydrogenase; HR: hazard ratio; ICU: intensive care unit; IFN: interferon; IMV: invasive mechanical ventilation; IQR: interquartile range; IU: international unit; MV: mechanical ventilation; NEWS2: National Early Warning Score 2; OSCI: ordinal scale for clinical improvement; OR: odds ratio; PCR: polymerase chain reaction; RCT: randomized controlled trial; rhG-CSF: Recombinant human granulocyte colony-stimulating factor; RR: relative risk; SD: standard deviation; SE: standard error; SOFA: sequential organ failure assessment; WHO: World Health Organization.
All of the presented studies were conducted in 2020. In the outcome description, the former is the data of the treatment group and the latter is the data of the control group. *The United States, Denmark, the United Kingdom, Greece, Germany, South Korea, Mexico, Spain, Japan, and Singapore. †The United States, Italy, Spain, Germany, Hong Kong, Singapore, South Korea, and Taiwan. ‡750 mg for participants with a mild or ordinary condition or 1,000 mg for participants with a severe or critical condition. §Defined as normalization of body temperature, respiratory rate, oxygen saturation, cough, and absorption of pulmonary infection on chest CT. ¶Resolving from fever to an axillary temperature of 36.6°C or below, normalization of SpO2 (> 94% on room air), and disappearance of respiratory symptoms including nasal congestion, cough, sore throat, sputum production, and shortness of breath. **The most common adverse event in the treatment group was diarrhea (7/70). ††Preprints from Medrxiv.org. ‡‡Defined as development of fever higher than 101 F for more than 72 hours, shortness of breath by minimal exertion (10-Step walk test), derangement of basic laboratory parameters (absolute lymphocyte count < 1,000 mm3 or raised serum C-reactive protein level), or appearance of infiltrates on chest radiograph during course of treatment. §§In the case of azithromycin coadministration, a single 1.0-mg loading dose of colchicine was administered. ¶¶Chloroquine or hydroxychloroquine was administered to 100% and 96% of participants in the treatment and the control group, respectively. Azithromycin was administered to 93% and 92% of participants in the treatment and the control group, respectively. ***Defined as a Borg score > 3, improved dyspnea, stopped fever for 72 hours, SO2 > 93%, tolerated oral regimen, normal urinary output, and reduced C-reactive protein level without any side effects. †††The dosage was determined according to age, body weight, and creatinine clearance. ‡‡‡The deferred treatment group received convalescent plasma only when a PaO2/FiO2 < 200 criterion was met during hospitalization or when the patient still required hospitalization for symptomatic COVID-19 more than 7 days after enrollment. §§§Progression to acute respiratory distress syndrome, sepsis, or septic shock.
Summary of meta-analyses evaluating therapeutics for COVID-19
| Comparisons | First author | No. of studies | No. of participants | Type of metrics | Model | Summary effect (95% CI) | P | I2 (P) | Publication bias | Conclusion | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiviral agents | ||||||||||||
| Remdesivir | ||||||||||||
| Mortality | Misra [22] | 2 [20, 21] | 0 | 54 (54)/696 (696) | 64 (64)/599 (599) | RR | Random | 0.74 (0.40 to 1.37) | NA | 58% (0.12) | NA | Not effective |
| Clinical recovery | Misra [22] | 2 [20, 21] | 0 | 437 (437)/696 (696) | 318 (318)/599 (599) | RR | Fixed | 1.17 (1.07 to 1.29) | NA | 0% (0.70) | NA | Effective |
| Clinical improvement* (5 vs. 10-days of treatment) | Jiang [26] | 2 [24, 25] | 0 | 263 (263)/391 (391) | 233 (233)/390 (390) | OR | Random | 1.33 (1.01 to 1.76) | NA | NA | NA | Favors 5-day treatment |
| Adverse events | Misra [22] | 2 [20, 21] | 0 | 258 (258)/696 (696) | 222 (222)/599 (599) | RR | Fixed | 0.91 (0.79 to 1.05) | NA | 7% (0.30) | NA | Inconclusive |
| Serious adverse events | Juul [186] | 2 [20, 21] | 0 | 142 (142)/ 554 (554) | 161 (161)/438 (438) | RR | Random | 0.77 (0.63 to 0.94) | 0.01 | 0.0% (0.66) | NA | Inconclusive |
| Favipiravir | ||||||||||||
| Clinical improvement by day 14 | Shrestha [187] | 2 [31, 188] | 1 | 73 (41)/84 (49) | 49 (21)/75 (30) | RR | Fixed | 1.29 (1.08 to 1.54) | 0.005 | 16% (0.30) | NA | Effective |
| Shrestha [187] | 2 [31, 188] | 0 | 41 (41)/49 (49) | 21 (21)/30 (30) | RR | Fixed | 1.12 (0.87 to 1.44) | 0.37 | 0% (0.98) | NA | Not effective | |
| Viral clearance by day 14 | Shrestha [187] | 2 [31, 188] | 1 | 77 (44)/84 (49) | 61 (28)/75 (30) | RR | Random | 1.06 (0.84 to 1.33) | 0.65 | 67% (0.05) | NA | Inconclusive |
| Shrestha [187] | 2 [31, 188] | 0 | 44 (44)/49 (49) | 28 (28)/30 (30) | RR | Random | 0.95 (0.74 to 1.22) | 0.67 | 41% (0.19) | NA | Inconclusive | |
| Umifenovir | ||||||||||||
| Clinical recovery | Misra [22] | 1 [34] | 1 | 51 (32)/69 (35) | 40 (13)/65 (17) | RR | Fixed | 1.08 (0.85 to 1.38) | NA | 0% (0.42) | N | Not effective |
| Viral clearance (on day 14) | Huang [35] | 1 [34] | 4 | 122 (32)/140 (35) | 174 (13)/247 (17) | RR | Random | 1.27 (1.04 to 1.55) | 0.02 | 63% (0.03) | NA | Possible effect |
| Adverse events | Misra [22] | 1 [34] | 1 | 8 (5)/69 (35) | 4 (0)/65 (17) | RR | Fixed | 1.80 (0.52 to 6.19) | NA | 10% (0.29) | N | Inconclusive |
| Lopinavir/ritonavir | ||||||||||||
| Clinical recovery | Misra [22] | 2 [34, 40] | 1 | 135 (107)/185 (133) | 110 (83)/165 (117) | RR | Fixed | 1.08 (0.94 to 1.24) | NA | 0% (0.70) | N | Not effective |
| Viral clearance | Wang [42] | 2 [34, 40] | 1 | 96 (61)/153 (93) | 141 (53)/209 (88) | RR | Fixed | 0.90 (0.76 to 1.07) | 0.225 | 33.9% (0.220) | N | Inconclusive |
| Liu [43] | 2 [34, 40] | 0 | 48 (48)/80 (80) | 45 (45)/78 (78) | RR | Random | 0.99 (0.76 to 1.29) | 0.93 | 0% (0.74) | NA | Inconclusive | |
| Adverse events | Misra [22] | 2 [34, 40] | 1 | 67 (58)/185 (133) | 53 (49)/165 (117) | RR | Random | 1.73 (0.57 to 5.26) | NA | 67% (0.05) | N | Inconclusive |
| Increased serum creatinine | Zhong [189] | 1 [40] | 1 | 4 (2)/147 (95) | 7 (7)/147 (99) | RR | Random | 0.86 (0.66 to 11.97) | NA | 61% (0.110) | NA | Inconclusive |
| Hydroxychloroquine | ||||||||||||
| 28-day mortality | Elsawah [190] | 2 [179, 180] | 0 | 0 (0)/239 (239) | 0 (0) 264 (264) | RD | Fixed | 0.00 (-0.01 to 0.01) | 1.00 | 0% (1.00) | NA | Not effective |
| Mortality | Yang [191] | 1 [192] | 4 | 91 (0)/451 (15) | 284 (0)/930 (15) | OR | Random | 1.23 (0.38 to 3.97) | 0.73 | 88% (<0.0001) | N | Not effective |
| Das [193] | 0 | 8 | 268 (0)/2009 (0) | 533 (0)/3671 (0) | OR | Random | 0.87 (0.46 to 1.64) | 0.66 | 92% (<0.00001) | Y | Not effective | |
| Thoguluva Chandrasekar [194] | 0 | 4 | 452 (0)/2111 (0) | 125 (0)/1041 (0) | OR | Random | 1.86 (1.38 to 2.50) | <0.001 | 29% (0.234) | NA | Harmful | |
| Zang [195] | 0 | 3 | 63 (0)/311 (0) | 27 (0)/268 (0) | RR | Fixed | 1.92 (1.26 to 2.93) | 0.003 | 0% (0.508) | NA | Harmful | |
| Deterioration† | Yang [191] | 3 [55, 180, 192] | 3 | 48 (2)/494 (116) | 29 (4)/540 (126) | OR | Random | 2.46 (0.42 to 14.45) | 0.32 | 69% (0.007) | N | Not effective |
| Liu [43] | 3 [55, 180, 192] | 0 | 2 (2)/115 (115) | 4 (4)/125 (125) | RR | Random | 0.96 (0.10 to 9.66) | 0.98 | 41% (0.8) | NA | Not effective | |
| Wang [42] | 2 [55, 192] | 3 | 244 (1)/843 (46) | 858 (4)/4112 (46) | RR | Random | 1.05 (0.61 to 1.81) | NA | 62.5% (0.031) | N | Not effective | |
| Clinical progression within 5-7 days‡ | Elsawah [190] | 2 [55, 192] | 2 | 11 (1)/89 (46) | 6 (4)/83 (46) | RD | Fixed | 0.06 (-0.03 to 0.15) | 0.18 | 76% (0.006) | NA | Not effective |
| Clinical progression within 28 days‡ | Elsawah [190] | 2 [179, 180] | 0 | 9 (9)/206 (206) | 11 (11)/234 (234) | RD | Fixed | -0.00 (-0.04 to 0.04) | 0.86 | 0% (0.33) | NA | Not effective |
| Death or invasive MV | Putman [196] | 0 | 2 | 166 (0)/895 (0) | 83 (0)/654 (0) | HR | Random | 1.03 (0.82 to 1.29) | 0.81 | 0% (0.75) | NA | Not effective |
| Death or deterioration† | Sarma [197] | 2 [55, 192] | 1 | 5 (1)/66 (46) | 4 (4)/62 (46) | OR | Random | 1.37 (0.09 to 21.97) | 0.82 | 59% (0.09) | NA | Not effective |
| Death or deterioration† (≤ 400 mg/day) | Yang [191] | 2 [55, 192] | 2 | 64 (1)/365 (46) | 270 (4)/787 (46) | OR | Random | 0.64 (0.14 to 2.81) | 0.55 | 84% (0.0002) | N | Not effective |
| Death or deterioration† (> 400 mg/day) | Yang [191] | 1 [180] | 1 | 5 (1)/90 (70) | 0 (0)/96 (80) | OR | Fixed | 6.17 (0.71 to 53.47) | 0.10 | 0% (0.67) | N | Not effective |
| Clinical recovery | Misra [22] | 2 [180, 192] | 5 | 1026 (69)/1474 (90) | 1054 (67)/1376 (90) | RR | Random | 0.93 (0.84 to 1.04) | NA | 74% (<0.01) | Y | Not effective |
| Talaie [198] | 2 [55, 180] | 0 | 70 (70)/106 (106) | 67 (67 )/106 (106) | RR | Random | 1.04 (0.85 to 1.28) | NA | 79.3% (0.028) | NA | Not effective | |
| Radiological improvement | Ullah [199] | 2 [55, 192] | 1 | 40 (30)/56 (46) | 33 (24)/58 (46) | OR | Random | 1.98 (0.47 to 8.36) | 0.36 | 54% (0.11) | N | Not effective |
| Radiological progression | Sarma [197] | 2 [55, 192] | 0 | 7 (7)/46 (46) | 16 (16)/46 (46) | OR | Random | 0.31 (0.11 to 0.90) | 0.03 | 16% (0.27) | NA | Not effective |
| Viral clearance | Singh [200] | 2 [180, 192] | 1 | 80 (72)/99 (85) | 81 (79)/111 (95) | RR | Random | 1.05 (0.79 to 1.38) | 0.744 | 62% (0.07) | Y | Inconclusive |
| Liu [43] | 2 [180, 192] | 0 | 77 (77)/90 (90) | 80 (80)/90 (90) | RR | Random | 0.98 (0.89 to 1.07) | 0.65 | 0% (0.54) | NA | Inconclusive | |
| Elavarasi [201] | 0 | 3 | 217 (0)/240 (0) | 152 (0)/203 (0) | RR | Random | 1.21 (0.64 to 2.29) | 0.56 | 87% (0.0006) | NA | Inconclusive | |
| Adverse events | Wang [42] | 3 [55, 180, 192] | 1 | 35 (27)/200 (116) | 10 (10)/223 (126) | RR | Fixed | 3.62 (1.93 to 6.79) | NA | 17.6% (0.303) | N | Possible harm |
| Zhong [189] | 3 [55, 180, 192] | 0 | 27 (27)/116 (116) | 10 (10)/126 (126) | RR | Random | 2.75 (1.42 to 5.33) | NA | 0% (0.442) | NA | Possible harm | |
| Adverse events (gastrointestinal) | Elsawah [190] | 3 [179, 180, 192] | 0 | 157 (157)/254 (254) | 7 (7)/279 (279) | RD | Fixed | 0.59 (0.55 to 0.64) | <0.00001 | 99% (<0.00001) | NA | Possible harm |
| Adverse events (CNS) | Elsawah [190] | 3 [55, 179, 180] | 0 | 65 (65)/270 (270) | 3 (3)/295 (295) | RD | Fixed | 0.23 (0.18 to 0.28) | <0.00001 | 99% (<0.00001) | NA | Possible harm |
| Adverse events (neurological) | Ullah [199] | 2 [180, 192] | 1 | 2 (2)/111 (101) | 2 (0)/123 (111) | OR | Random | 1.26 (0.20 to 7.98) | 0.81 | 0% (0.37) | N | Inconclusive |
| Adverse events (cardiac) | Elsawah [190] | 2 [179, 180] | 0 | 3 (3)/239 (239) | 0 (0)/264 (264) | RD | Fixed | 0.01 (-0.01 to 0.03) | 0.16 | 84% (0.01) | NA | Inconclusive |
| Hydroxychloroquine plus azithromycin | ||||||||||||
| Mortality | Das [193] | 0 | 4 | NA (0)/1145 (0) | NA (0)/1165 (0) | OR | Random | 2.84 (2.19 to 3.69) | <0.00001 | 0% (0.43) | Y | Harmful |
| Yang [191] | 0 | 3 | 214 (0)/854 (0) | 46 (0)/395 (0) | OR | Fixed | 2.34 (1.63 to 3.36) | <0.00001 | 0% (0.85) | N | Harmful | |
| Deterioration† | Yang [191] | 0 | 3 | 101 (0)/840 (0) | 25 (0)/414 (0) | OR | Random | 4.97 (0.01 to 4781.7) | 0.65 | 95% (<0.00001) | N | Not effective |
| Wang [42] | 0 | 2 | 115 (0)/328 (0) | 833 (0)/3969 (0) | RR | Random | 0.93 (0.17 to 5.09) | NA | 94.2% (<0.001) | N | Not effective | |
| Corticosteroids | ||||||||||||
| Mortality | Lu [67] | 0 | 4 | 94 (0)/329 (0) | 58 (0)/408 (0) | RR | Random | 2.00 (0.69 to 5.75) | NA | 90% (<0.001) | NA | Not effective |
| Mortality (severe COVID-19 subgroup) | Ye [68] | 0 | 2 | NA (0)/227 (0) | NA (0)/104 (0) | HR | Random | 2.30 (1.00 to 5.29) | NA | 0% (0.768) | NA | Not effective |
| Time to viral clearance | Sarkar [69] | 0 | 2 | 82 | 69 | MD | Random | 1.42 (-0.52 to 3.37) | 0.15 | 0% (0.52) | NA | Not effective |
| Renin-angiotensin-aldosterone system inhibitors for patients with hypertension | ||||||||||||
| Mortality (ACEI) | Pranata [73] | 0 | 3 | 29 (0)/110 (0) | 87 (0)/326 (0) | OR | Random | 0.68 (0.39 to 1.17) | 0.16 | 0% (0.62) | Y | Not effective |
| Mortality (ARB) | Pranata [73] | 0 | 3 | 29 (0)/158 (0) | 87 (0)/326 (0) | OR | Random | 0.51 (0.29 to 0.90) | 0.02 | 22% (0.28) | Y | Effective |
| Mortality (ACEI or ARB) | Flacco [74] | 0 | 4 | NA (0)/921 (0) | NA (0)/1491 (0) | OR | Random | 0.88 (0.68 to 1.14) | 0.33 | 24% (0.27) | N | Not effective |
| Anticoagulants | ||||||||||||
| Mortality | Lu [76] | 0 | 5 | 536 (0 )/2886 (0) | 947 (0)/5647 (0) | RR | Random | 0.86 (0.69 to 1.09) | 0.218 | 47.4% (0.107) | NA | Not effective |
| Heparin - mortality (severe COVID-19 subgroup) | Abdel-Maboud [77] | 0 | 2 | 50 (0)/126 (0) | 115 (0)/368 (0) | RR | Random | 1.09 (0.84 to 1.42) | NA | 0% (0.537) | NA | Not effective |
| Convalescent plasma | ||||||||||||
| Mortality | Talaie [198] | 1 [87] | 2 | 10 (8)/82 (52) | 21 (12)/81 (51) | RR | Random | (0.26 to 1.03) | NA | 0% (0.484) | N | Not effective |
| Clinical improvement | Talaie [198] | 1 [87] | 2 | 46 (27)/82 (52) | 32 (22)/81 (51) | RR | Random | 1.41 (1.01 to 1.98) | NA | 66.6% (0.050) | Y | Effective |
| Viral clearance | Sarkar [202] | 1 [87] | 2 | 54 (41)/68 (52) | 18 (15)/76 (51) | OR | Random | 11.29 (4.92 to 25.92) | <0.00001 | 0% (0.40) | Y | Possible effect |
| Tocilizumab | ||||||||||||
| Mortality | Lan [203]§ | 0 | 7 | 39 (0)/241 (0) | 85 (0)/352 (0) | RR | Random | 0.61 (0.31 to 1.22) | 0.16 | 68% (0.005) | NA | Not effective |
| Mortality (lopinavir/ritonavir subgroup)¶ | Malgie [94] | 0 | 2 | 7 (0)/94 (0) | 22 (0)/56 (0) | RD | Random | -0.31 (-0.57 to -0.05) | NA | NA | Y | Effective |
| ICU admission and initiation of MV | Lan [203]§ | 0 | 5 | 47 (0)/134 (0) | 44 (0)/279 (0) | RR | Random | 1.51 (0.33 to 6.78) | 0.59 | 86% (<0.00001) | NA | Not effective |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARDS: acute respiratory distress syndrome; CI: confidence interval; CNS: central nervous system; COVID-19: coronavirus disease 2019; ECG: electrocardiogram; HR: hazard ratio; ICU: intensive care unit; MD: mean difference; MV: mechanical ventilation; NA: not applicable; OR: odds ratio; RCT: randomized controlled trial; RD: risk difference; RR: relative risk.
The RCTs included in the meta-analyses of this table were also included in our target RCTs and are presented in Table 3, except an RCT [25] with no peer-reviewed or preprint report released, an RCT [192] published in Chinese, and an RCT [188] in which a statistical analysis was not conducted. *A 2-point reduction on a 7-category ordinal scale. †Progression to severe COVID-19. ‡An increase in severity compared to the baseline severity. §One participant in the treatment arm of one included study was diagnosed as suspected COVID-19 with a negative PCR result. ¶All participants received lopinavir plus ritonavir but did not receive corticosteroids.
Lopinavir/ritonavir
In two human non-RCTs on SARS, the treatment group performed better with respect to the overall mortality rate or the incidence of ARDS [36, 37]. An ongoing RCT on MERS involved a combination of lopinavir/ritonavir and IFN-β [19], which has been shown to be effective in two in vivo studies on MERS [38, 39] (Table 1). In an RCT on COVID-19, treatment with lopinavir/ritonavir was not associated with a mortality rate reduction at day 28 (treatment group 19.2% vs. control group 25.0%; difference, -5.8%; 95% CI -17.3% to 5.7%) [40]. In the aforementioned RCT on COVID-19 comparing a combination of lopinavir/ritonavir and umifenovir with standard treatment [34], and in another RCT on COVID-19 comparing “lopinavir/ritonavir plus IFN-α with or without ribavirin” with “ribavirin plus IFN-α” [41], treatment with lopinavir/ritonavir did not show superior outcomes in terms of clinical deterioration or viral clearance (Table 3). In meta-analyses on COVID-19 involving two of these RCTs [34, 40], treatment with lopinavir/ritonavir was not associated with clinical recovery or viral clearance [22, 42, 43] (Table 4).
Ribavirin
Ribavirin has been investigated in previous studies on SARS and MERS, but the results were not consistent (Table 1). Although one RCT for a combination therapy of ribavirin and lopinavir/ritonavir in SARS was registered [18], it seems unlikely that this trial can be finished, as SARS has not occurred for a while. In two in vitro studies on COVID-19, ribavirin did not show therapeutic effects [29, 30] (Table S1). In the aforementioned RCT on COVID-19, comparing “ribavirin plus interferon IFN-α with or without lopinavir/ritonavir” with “lopinavir/ritonavir plus IFN-α”, treatment with ribavirin did not show better outcome in terms of the clinical deterioration or viral clearance [41]. In another RCT on COVID-19, a combination of ribavirin and IFN-β1b was evaluated: In the treatment group, the time taken to achieve a National Early Warning Score 2 (NEWS2) (4 [IQR 3-8] vs. 8 [7-9] days; HR 3.92, 95% CI 1.66 to 9.23) or sequential organ failure assessment (SOFA) score of zero (3.0 [IQR 1.0-8.0] vs. 8.0 [6.5-9.0] days; HR 1.89, 95% CI 1.03 to 3.49), the hospitalization period (9.0 [IQR 7.0-13.0] vs. 14.5 [9.3-16.0] days; HR 2.72, 95% CI 1.2 to 6.13), and time to viral clearance (7 [IQR 5-11] vs. 12 [8-15] days; HR 4.37, 95% CI 1.86 to 10.24, P = 0.001) were shorter than those of the control group [44] (Table 3).
Other antiviral agents
In a small-scale RCT on mild to moderate COVID-19, azvudine (FNC) treatment showed better outcome with respect to radiological improvement (P = 0.0401) and viral clearance (P = 0.0011) [45]. In the other RCTs on COVID-19, triazavirin [46] or a combination of darunavir and cobicistat [47] did not show therapeutic effects (Table 3). Nelfinavir showed therapeutic effects in one [48] out of two in vitro studies on SARS (Table 1) and in an in vitro study on COVID-19 [49] (Table 2).
4-Aminoquinoline
In a study on SARS, chloroquine showed an effect in vitro but not in vivo, and these results were similar for amodiaquine [50]. The results of two in vitro studies of chloroquine for MERS conflicted with each other [51, 52]. Multiple in vitro studies on COVID-19 reported effects of chloroquine [29, 53, 54] and hydroxychloroquine [53, 54]. In a preprint RCT on COVID-19, hydroxychloroquine was administered with a daily dosage of 400 mg for five consecutive days and the treatment group showed higher rates of improvement in chest computed tomography (CT) scans on day 6 (25 out of 31 [81%] vs. 17 out of 31 [55%], P = 0.0476) and shorter duration of fever (2.2 [standard deviation (SD) 0.4] vs. 3.2 [1.3] days, P = 0.0008) [55]. In another preprint RCT conducted in Pakistan enrolling 500 patients with mild COVID-19, the proportion of patients with negative viral PCR results within seven days was higher in the hydroxychloroquine-treated group (182 out of 349 [52%] vs. 54 out of 151 [36%], P = 0.001) [56]. However, in the other four RCTs and one preprint RCT on COVID-19 involving a total of 815 participants, treatment with hydroxychloroquine did not show better outcome compared to standard treatment (Table 3). In 15 meta-analyses on COVID-19, treatment with hydroxychloroquine showed no therapeutic effect and higher risk for adverse events. A combination of hydroxychloroquine and azithromycin was also evaluated in three meta-analyses on non-RCTs for COVID-19 and showed a harmful effect (Table 4).
Azithromycin
In an RCT on azithromycin for COVID-19, the hospitalization period of the treatment group was shorter than that of the control group (4.6 [SD 2.6] vs. 6.0 [SD 3.2] days, P = 0.02) [57]. However, in another RCT on azithromycin involving 397 patients with severe COVID-19, azithromycin did not show any therapeutic effect [58] (Table 3).
Corticosteroids
In an RCT targeting SARS, early administration (within 7 days) of corticosteroids was associated with higher subsequent plasma viral concentrations in the second and third week of the illness [59]. However, in this study, the severity of disease did not differ between the early corticosteroid treatment group and the control group. In addition, there was no significant difference in the median time for the virus to become undetectable in plasma between the early corticosteroid treatment group and the control group (12 vs. 8 days, P = 0.106). Therefore, it is difficult to conclude that this study supports the risk of corticosteroid treatment. A non-RCT on SARS demonstrated that corticosteroid therapy was associated with higher risk for either ICU admission or mortality (OR 20.7, 95% CI 1.3-338.0) [60]. This study had several important limitations, including the following: (1) the 95% CI was extremely asymmetric; (2) there was no difference in mortality between the steroid-treated and non-treated groups in a simple univariate analysis, but corticosteroid therapy was included in the logistic regression; (3) the steroid-treated group had a more severe disease course, which indicates a case of confounding by indication; and (4) not all of the potential variables were adjusted, which could influence the results.
In a non-RCT on MERS, corticosteroid therapy was not associated with 90-day mortality but associated with delay in viral clearance (adjusted HR 0.35, 95% CI 0.17-0.72) under a marginal structural model [61]. However, this study also shared many of the shortcomings mentioned above such as corticosteroids being used for patients with severe conditions, which can introduce severe levels of bias. In this retrospective study, at least a propensity-score matching analysis should have been considered.
Three non-RCTs of corticosteroid use in SARS showed effectiveness of high dose [62-64]. One non-RCT on SARS demonstrated that the survival outcome of the group receiving methylprednisolone was superior compared to the group not receiving corticosteroids as well as the group receiving hydrocortisone or pulse therapy [65]. In an RCT on methylprednisolone treatment for severe COVID-19, the treatment group showed a lower mortality rate (2 out of 34 [6%] vs. 12 out of 28 [43%], P < 0.001) and a higher rate of clinical improvement (32 out of 34 [94%] vs. 16 out of 28 [57%], P = 0.001) compared to the control group [66]. Meta-analyses on corticosteroid therapy for COVID-19 included only non-RCTs and did not demonstrate any significant therapeutic effect of corticosteroids [67-69] (Table 4).
Colchicine
In two RCTs on COVID-19, colchicine showed effects in major outcomes. Among them, in a Greek RCT, the treatment group had a higher cumulative event-free 10-day survival rate (97% vs. 83%, P = 0.03), a longer event-free survival period (21 [SD 0.31] vs. 19 [0.83] days, P = 0.03), and a lower incidence of deterioration within three weeks (2% vs. 14%; OR 0.11, 95% CI 0.01 to 0.96; P = 0.046) [70]. In another preprint RCT conducted in Brazil, the treatment group had a shorter duration of supplemental oxygen therapy (3.0 [IQR 1.5-6.5] vs. 7.0 [3.0-8.5] days, P = 0.02), a lower proportion of participants requiring supplemental oxygen on day 7 (6% vs. 39%, P = 0.01), a shorter length of hospital stay (6.0 [IQR 4.0-8.5] vs. 8.5 [5.5-11.0] days, P = 0.03), and lower rate of hospitalization (53% vs. 78% on day 5; 6% vs. 17% on day 10; P = 0.01) [71].
ACEI or ARB
In a preprint RCT on 82 participants with COVID-19, telmisartan was administered to the treatment group with a daily dosage of 160 mg for 14 consecutive days. The treatment group had a shorter duration of hospital stay (9 vs. 15 days, P = 0.0124) and the HR for hospital discharge was 2.02 (95% CI 1.14 to 3.59) [72] (Table 3). In a meta-analysis that included three non-RCTs on COVID-19 with hypertension, ARB showed a survival benefit (OR for mortality 0.51, 95% CI 0.29 to 0.90, P = 0.02; I2 = 22%, n = 484) although there was a publication bias [73]. In another meta-analysis that included non-RCTs on COVID-19, the effect of ACEI or ARB therapy on COVID-19 with hypertension was not significant [74] (Table 4).
Anticoagulants
In a small-scale RCT for severe COVID-19, therapeutic anticoagulant therapy with enoxaparin and prophylactic anticoagulant therapy with enoxaparin or unfractionated heparin were compared. A greater proportion of participants in the therapeutic anticoagulant group were able to be weaned from MV successfully compared to the prophylactic anticoagulant group (8 out of 10 [80%] vs. 3 out of 10 [30%]; HR 4.0, 95% CI 1.04 to 15.05; P = 0.031) [75] (Table 3). In two meta-analyses including non-RCTs on COVID-19, anticoagulant therapy did not show a therapeutic effect [76, 77] (Table 4).
Calcifediol
Calcifediol was studied in one RCT on COVID-19. In this RCT, a lower proportion of participants in the treatment group were admitted to the ICU compared to the control group (1 out of 50 [2%] vs. 13 out of 26 [50%]; adjusted OR 0.03, 95% CI 0.003 to 0.25) [78] (Table 3).
CM4620-IE (AuxoraTM, calcium release-activated calcium channel inhibitor)
In an RCT on severe or critical COVID-19, the proportion of patients who received invasive MV or died was lower in the Auxora-treated group than the control group (3 out of 17 [18%] vs. 5 out of 9 [6%]; HR 0.23, 95% CI 0.05 to 0.96; P < 0.05) and the mean difference in the 8-point ordinal scale was statistically significant on day 6 and day 9 to 12 (P < 0.05) [79] (Table 3).
Janus-associated kinase inhibitors
In an in vitro study on MERS, baricitinib showed an effect [80] (Table 1). Ruxolitinib was evaluated in an RCT on severe COVID-19 and showed higher rates of improvement on chest CT scans on day 14 (18 out of 20 [90%] vs. 13 out of 21 [62%], P = 0.0495) and shorter time to lymphocyte recovery (5 [IQR 2-7] vs. 8 [2-11] days, P = 0.033) [81] (Table 3).
Leflunomide (dihydroorotate dehydrogenase inhibitor)
In a small-sized RCT on moderate COVID-19, the duration of viral shedding was shorter in the leflunomide-treated group compared with the control group (5 vs. 11 days, P = 0.046) [82] (Table 3).
Immunotherapy
Interferon
Both IFN-α and IFN-β showed numerous positive results in non-human studies on SARS or MERS. However, a meta-analysis on MERS did not show that the interferon therapy was effective [14]. For COVID-19, five RCTs on interferon have been published to date. In an RCT evaluating IFN-β1a treatment, the rate of hospital discharge was higher (67% vs. 44%; OR 2.5, 95% CI 1.05 to 6.37) and the 28-day mortality rate was lower (19% vs. 44%, P = 0.015) in the treatment group compared to the control group [83]. In the aforementioned RCT evaluating a combination of IFN-β1b and ribavirin, the treatment group performed better [44]. In another RCT on severe COVID-19, treatment with IFN-β1b showed better outcomes in terms of discharge from the hospital and admission to the ICU compared to standard treatment [84]. Inhaled IFN-κ plus TFF2 therapy was investigated in one RCT on moderate COVID-19. In this study, the chest CT findings of participants in the treatment group improved within a shorter time compared to the control group (6.2 [95% CI 5.1-7.3] vs. 8.8 [95% CI 7.6-10.0] days, P = 0.002) [85]. In an RCT evaluating Novaferon therapy, the group receiving the combination of Novaferon and lopinavir/ritonavir had a higher rate of viral clearance on day 6 (18 out of 30 [60%] vs. 7 out of 29 [24%], P = 0.0053) and a shorter median time to negative results of virus PCR (6 vs. 9 days, P = 0.036) compared with the group receiving lopinavir/ritonavir [86] (Table 3).
Convalescent plasma
A meta-analysis of convalescent plasma therapy in patients with SARS demonstrated that the absolute reduction in the risk of mortality was 7% and 23% in two studies, and the case fatality rate from four non-comparative studies varied from 0% to 12.5% [12]. In an RCT for severe or life-threatening COVID-19, the proportion of participants who recovered clinically within 28 days was higher in the treatment than in the control group in cases with severe COVID-19 (21 out of 23 [91%] vs. 15 out of 22 [28%]; HR 2.15, 95% CI 1.07 to 4.32) [87]. Four preprint RCTs on COVID-19 have investigated the effect of convalescent plasma. In one of these RCTs, a lower proportion of participants in the treatment group compared to the control group either required MV or died (0 out of 38 [0%] vs. 6 out of 43 [14%], P = 0.03) [88]. Another RCT on moderate COVID-19 involving more participants (n = 464) did not show the therapeutic effect of convalescent plasma [89] (Table 3).
Recombinant human granulocyte colony-stimulating factor (rhG-CSF)
In an RCT for COVID-19 with lymphopenia, participants in the treatment group who received rhG-CSF showed lower rates of 21-day mortality (2 out of 100 [2%] vs. 10 out of 100 [10%]; HR 0.19, 95% CI 0.04 to 0.88) and disease progression (2 out of 100 [2%] vs. 15 out of 100 [15%]; mean difference -13%, 95% CI -21.4% to -5.4%) [90] (Table 3).
Intravenous immunoglobulin
In a preprint RCT on COVID-19, the 3-day course of intravenous immunoglobulin therapy showed a lower rate of MV within 30 days (2 out of 14 [14%] vs. 7 out of 12 [58%], P = 0.038), shorter hospital stay (11 [range 5-22] vs. 19 [4-30] days, P = 0.013) or ICU stay (2.5 [range 0-16] vs. 12.5 [1-29] days, P = 0.006), and improvement in PaO2/FiO2 ratio on day 7 (difference +131 [+35 to +330] vs. +44.5 [-115 to +157], P = 0.01) among the participants who had an alveolar-arterial oxygen gradient greater than 200 mmHg at enrollment [91] (Table 3).
Other immunotherapies
In a small-scale RCT on severe COVID-19, vilobelimab (anti-C5a antibody IFX-1) treatment did not show a therapeutic effect [92]. In a small-scale RCT on CIGB (anti-CK2) for COVID-19, there was a reduction in the number of pulmonary lesions on chest CT in a greater proportion of participants in the treatment group compared to the control group (5 out of 6 [83%] vs.3 out of 7 [43%]; Bayesian P (difference > 0) = 0.951) [93]. Multiple observational clinical studies on tocilizumab (anti-interleukin [IL] -6 receptor antibody) for COVID-19 were investigated in two meta-analyses. Among them, a subgroup analysis, in which lopinavir and ritonavir were and corticosteroids were not administered to all participants, showed a lower mortality rate in the tocilizmub treatment group (risk difference -0.31, 95% CI -0.57 to -0.05) [94] (Table 4).
Discussion
We summarized the results of studies conducted on SARS, MERS, and COVID-19 to date. Unfortunately, completed RCTs for the treatment of SARS and MERS were scarce. We assumed this was because SARS was a relatively short-lived epidemic that has not occurred since 2004, and the number of patients with MERS might have been insufficient for recruitment. In the case of COVID-19, numerous RCTs have been registered, and research results have been consistently reported despite the global pandemic and medical crisis.
It was difficult to find an optimal therapeutic agent that consistently resulted in positive outcomes across SARS, MERS, and COVID-19. One of the possible reasons of this is that there might not be a universal “cure” to these viral diseases given the differences in presentation forms. Reduction of the viral load may not be the only aim when attempting to cure the disease. The subtle differences between these three coronaviruses, as well as the lack of objective information from clinical experiences of the preceding SARS and MERS epidemics, may also be other reasons.
Synthesizing studies on COVID-19 highlighted two main goals in the treatment of COVID-19: (1) effective elimination of the virus and (2) immune regulation to interfere with the mechanisms of cytokine storm. Therefore, extensive further research on various antiviral agents and immunomodulators is expected to continue for a while.
Among the antiviral agents presented in our study, remdesivir has consistently shown potent effects in non-human studies on SARS, MERS, and COVID-19. Remdesivir is a novel broad-spectrum antiviral agent. When remdesivir is administered into the human body, it is metabolized to an active metabolite, which is an adenosine nucleoside triphosphate analogue. It interferes with the action of viral RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), which interferes with RNA replication of the virus [95]. This agent showed an effect in the largest of the four RCTs on COVID-19 that included 1,062 participants. In this RCT, the 14-day mortality rate was significantly lower in the subgroup with requirement of only supplemental oxygen not MV, suggesting that there is a patient population with a certain level of severity of COVID-19 that could benefit from remdesivir treatment [20]. Therefore, it seems necessary to preferentially administer remdesivir to this specific group of patients. In addition, it should be considered in the clinical application that the outcome of 5-day regimen treatment was better than that of the 10-day regimen in two RCTs [23, 24]. On October 22, 2020, the Food and Drug Administration (FDA) approved the use of remdesivir for treatment of COVID-19 requiring hospitalization.
A combination of sofosbuvir and daclatasvir consistently showed an effect on the major outcomes in two RCTs on COVID-19. Sofosbuvir is a nucleotide analog targeting the hepatitis C virus (HCV) polymerase, NS5B. This agent is capable of inhibition of positive-strand RNA viruses like coronavirus. Daclatasvir is an HCV NS5A antagonist and is known to penetrate lung tissue effectively. Daclatasvir has been shown to inhibit the production of SARS-CoV-2 particles in an in vitro study [96]. Those two RCTs investigating the combination of sofosbuvir and daclatasvir had limitations because they included a small number of participants and did not show significant results for mortality. Therefore, a large-scale RCT on this treatment is still required.
Favipiravir is an antiviral agent that is drawing attention as a treatment option for COVID-19. The active metabolite of favipiravir competes with purine nucleosides and incorporates into viral RNA to interfere with viral replication, potentially inhibiting the RNA dependent RNA polymerase (RdRp) of RNA viruses [97]. Considering that favipiravir showed an effect in terms of viral clearance in an RCT on COVID-19 [31], a large-scale, well-designed RCT is further required. A number of RCTs on favipiravir are now underway.
Umifenovir is a small indole-derivative molecule with broad-spectrum antiviral property, and it has been approved in Russia and China for the prophylaxis and treatment of influenza. It inhibits the virus from fusion to the target cell membrane and blocks viral entry into the cell [98]. Since a meta-analysis including one RCT and multiple observational studies on umifenovir for COVID-19 demonstrated significant results for viral clearance [35], well-designed RCTs on umifenovir still needs to be conducted.
Lopinavir/ritonavir combination therapy seems to have more therapeutic effect than monotherapy of each drug. As protease inhibitors, lopinavir and ritonavir can inhibit the action of 3CLpro, coronavirus main protease, and interfere with the process of viral replication and release [99]. However, the clinical results of this combination therapy for COVID-19 were not meeting expectations compared to those of SARS and MERS. In a first RCT on lopinavir/ritonavir for COVID-19, the outcomes of the treatment group and the control group did not show a significant difference, but the 28-day mortality of the treatment group were slightly lower [40]; and in two other RCTs and four meta-analyses on COVID-19, treatment with lopinavir/ritonavir did not show an effect.
Azvudine (FNC) is a novel nucleoside reverse transcriptase inhibitor targeting HCV and has been investigated for treatment of human immunodeficiency virus (HIV) [100]. Azvudine showed promising effects in terms of radiological improvement and viral clearance in a small-scale RCT for mild to moderate COVID-19 [45]. RCTs involving more participants are required.
The 4-aminoquinoline is mainly used as an anti-malaria agent, and hydroxychloroquine, one of its derivatives, is also used as an immunomodulatory agent. Chloroquine and hydroxychloroquine inhibits the pH-dependent steps of viral replication by increasing the pH of phagolysosome [101]. The study on SARS showed in vitro but not in vivo effects of chloroquine and amodiaquine [50], and combination therapy with other drugs that inhibit viral replication may be necessary. In our study, we investigated seven RCTs and 15 meta-analyses on hydroxychloroquine for COVID-19 and only one preprint small-scale RCT demonstrated an effect of hydroxychloroquine on the major outcomes [55]. The survival benefit of hydroxychloroquine treatment has not been demonstrated in any of these seven RCTs. The results of the latest large-scale RCT [102], which was excluded from our study since it included patients with suspected COVID-19, as well as multiple meta-analyses were consistent with this. In addition, the risk for adverse events of hydroxychloroquine treatment was higher compared to standard treatment according to the results of meta-analyses. Therefore, hydroxychloroquine seems to have no value as a therapeutic agent for COVID-19.
It is worth noting that an RCT for chloroquine, excluded due to involvement of patients with suspected COVID-19 [103], suggested the risk of high-dose chloroquine treatment and this risk was associated with prolongation of the QT interval. Similarly, hydroxychloroquine treatment was also related to QT prolongation [104, 105]. Therefore, it is expected that if careful monitoring for prolongation of the QT interval is not accompanied, chloroquine or hydroxychloroquine treatment can be rather harmful. In this regard, the World Health Organization (WHO) recently decided to implement the temporary pause of the hydroxychloroquine arm within the Solidarity Trial, a large-scale study on four untested treatments for COVID-19 [106]. The FDA also withdrew emergency use authorization for chloroquine and hydroxychloroquine [107].
For severe inflammatory diseases caused by infection, corticosteroid therapy is a double-edged sword. Although a number of corticosteroid therapies have already been used in SARS, MERS, and COVID-19, these results are controversial and difficult to interpret. It is noteworthy that the study of SARS showed a difference in outcomes depending on the type or dosage of steroids [65]. In most observational studies on COVID-19, corticosteroid therapy was mainly administered in a group with severe clinical conditions according to the prevailing guidelines [7]. Because of this strong tendency, patients with COVID-19 who received corticosteroids had poor treatment outcomes, and objective validation of corticosteroid treatment has been highly required.
In response to this request, the results of the first RCT to investigate corticosteroid therapy in COVID-19 were recently reported, although it was excluded from our study because participants with negative SARS-CoV-2 PCR results were included. This RCT involved 2,104 and 4,321 participants in the treatment and control group, respectively. The 28-day mortality rate of the group receiving 10 days of dexamethasone treatment was lower than that of the control group (482 out of 2,104 [23%] vs. 1,110 out of 4,321 [26%]; RR 0.83, 95% CI 0.75 to 0.93; P < 0.001), and this trend was stronger among the subgroup with higher severity of COVID-19: (1) 95 out of 324 patients (29%) in the treatment group and 283 out of 683 patients (41%) in the control group among patients requiring invasive MV (RR 0.64, 95% CI 0.51 to 0.81) (2) 298 out of 1,279 patients (23%) in the treatment group and 68 out of 2,604 patients (26%) in the control group among patients requiring only oxygen supplement (RR 0.82, 95% CI 0.72 to 0.94). There was no significant difference in the 28-day mortality rate among patients not receiving respiratory support [108]. These results strongly suggest that dexamethasone treatment is effective for a population of patients with COVID-19 requiring respiratory support. It is necessary to confirm these results among the participants who were diagnosed as COVID-19 through viral RNA detection. In another RCT for severe COVID-19, patients receiving methylprednisolone also showed a lower mortality rate compared to the control group [66].
Colchicine, an anti-inflammatory agent which is mainly used for gout and rheumatoid arthritis, has been used for a long time. It inhibits microtubule polymerization and mitosis in the metaphase [109]. It is promising that the effects of colchicine treatment were revealed in terms of survival, clinical improvement, and duration of hospitalization in the two RCTs for COVID-19 [70, 71]. If a larger-scale follow-up RCT is conducted, the effect might be further supported.
Angiotensin-converting enzyme 2 (ACE2) is a transmembrane protein and the main entry point into cells for SARS-CoV-2. Theoretically, if the expression of ACE2 decreases, it will be a defense mechanism against the entry of the virus. On the other hand, ACE2 shows a protective action against virus-induce lung injury by converting angiotensin II to angiotensin-(1-7), which have a vasodilator effect [110, 111]. ACEI and ARB can induce up-regulation of ACE2 [112, 113], which might negatively affect the treatment of disease. Contrary, an in vivo study showed that SARS-CoV spike-mediated lung injury was attenuated by losartan [114]. For these conflicting evidences, there has been an interest in ACEI and ARB in relation to COVID-19. In one RCT for COVID-19 [72] and one meta-analysis including three non-RCTs on COVID-19 [73], participants who received ARB performed better in terms of discharge or survival. More RCTs on ACEI or ARB for COVID-19 in the group of patients with pre-existing hypertension or at risk for cardiovascular disease are still required.
Routine administration of anticoagulants in sepsis or ARDS is not recommended currently. However, disseminated intravascular coagulation should still be the target of research to find treatments for sepsis or ARDS, because it is deeply involved in the pathogenesis and progress of these diseases. It has been reported that coagulopathy was associated with the prognosis of COVID-19 [115-117] and these results are consistent with what has been known in ARDS and sepsis. In a small-scale RCT of severe COVID-19, therapeutic anticoagulant therapy with enoxaparin showed a better outcome than prophylactic anticoagulant therapy [75]. More studies investigating the efficacy of augmenting these anticoagulation or thrombolytic treatments, while weighing the risk of hemorrhage, and narrowing the indications are required.
Calcifediol is a main metabolite of vitamin D. Since lung epithelium expresses vitamin D receptors, administration of vitamin D may suppress the development of ARDS [118]. In a pilot RCT on COVID-19, treatment with a high dosage of calcifediol reduced the need for ICU admission [78].
CM4620-IE (AuxoraTM) is a selective small molecule inhibitor of calcium release-activated calcium (CRAC) channels. It was developed to prevent over-activation of CRAC channels that can lead to inflammatory diseases. It has been suggested that Auxora may protect against pulmonary endothelial damage and cytokine storm [119, 120]. This agent has been shown to be effective in terms of survival and clinical improvement in one small-scale RCT for severe or critical COVID-19 [79].
Ruxolitinib is a potent selective inhibitor of Janus-associated kinases 1 and 2 and has been used as a treatment for primary myelofibrosis, post-polycythemia vera or post-essential thrombocythemia myelofibrosis [121]. Ruxolitinib has a broad-spectrum of anti-inflammatory properties against cytokine storm mediated by IL-1, IL-6, IL-8, IL-12, tumor necrosis factor-α, IFN-γ, vascular endothelial growth factor, and granulocyte-macrophage colony-stimulating factor [122]. In an RCT on severe COVID-19, ruxolitinib showed higher rate of radiological improvement despite the small number of participants [81]. In this RCT, no one out of 20 patients with severe COVID-19 in the treatment group died within 28 days, whereas three out of 21 patients in the control group died. Further research results for severe COVID-19 at high risk for cytokine storm need to be supplemented.
Leflunomide, an isoxazole derivative, inhibits the T cell proliferation by blocking dihydroorotate dehydrogenase. This agent has been used in the treatment of rheumatoid arthritis and psoriatic arthritis, and has been attempted to treat BK virus, cytomegalovirus, HIV, and ebolavirus [123]. The only RCT evaluating leflunomide for COVID-19 enrolled only 10 participants, but showed an effect of shortening the viral shedding period [82]. Similarly, in a preprint observational study involving 27 participants, leflunomide showed effects of promoting viral clearance and increasing discharge rate [124].
Interferon showed effects in a number of in vitro studies on SARS and MERS. In addition, three RCTs on IFN-β for COVID-19 demonstrated favorable results in terms of survival, clinical improvement, discharge from hospital, and viral clearance [44, 83, 84]. Since interferon has been used as a combination therapy with antiviral agents in most cases, further research is needed to discover the antiviral agent that can show the greatest effect when administered in combination with interferon, as well as specific indications.
Convalescent plasma contains pathogen-specific neutralizing antibodies that can neutralize viral particles, which provide passive immunity to the recipient. It is hypothesized that early convalescent plasma therapy enhances the patient's capability to clear the initial viral inoculum by neutralizing viral particles [125]. Convalescent plasma therapy has been applied to a wide range of infectious diseases such as diphtheria, pneumococcal pneumonia, hepatitis A and B, mumps, polio, measles, and rabies. The results of a meta-analysis on convalescent plasma treatment for SARS are relatively promising [12]. In one RCT for severe COVID-19, convalescent plasma treatment showed an effect in terms of clinical improvement [87]. In contrast, the effect of convalescent plasma treatment was not demonstrated in another RCT on moderate COVID-19 involving larger number of participants [89]. The differences in severity of COVID-19 in these two RCTs may have contributed to this contradiction. Therefore, a large-scale RCT on convalescent plasma treatment targeting severe COVID-19 is required.
Intravenous immunoglobulin therapy provides passive immunity and has the property to modulate immune function. High doses of intravenous immunoglobulin can produce anti-inflammatory and inflammatory-modulating effects on a variety of immune cells, which can intervene and modulate the mechanisms of cytokine storm, and have been administered to treat various diseases such as immune thrombocytopenia purpura or Kawasaki disease [126]. The only preprint small-scale RCT on intravenous immunoglobulin therapy for COVID-19 showed better clinical outcomes in the treatment group [91].
Our study has some limitations. RCTs for SARS and MERS were extremely rare. In the case of COVID-19, more RCTs were obtained. However, all except for eight RCTs included less than 100 participants per each arm. Although we updated the latest search results for RCTs and meta-analyses on COVID-19, we did not include the latest search results for non-clinical studies on the three coronavirus diseases because of the vast amount of data.
In addition, our study also highlights that treatments with potential effects seen in in vitro studies have not translated in positive in vivo or clinical studies. The 4-aminoquinoline derivatives showed effects in a number of in vitro studies, but not in in vivo and clinical studies. On the other hand, favipiravir showed unfavorable results in an in vitro study, while it showed effects in a clinical study. This contradiction between in vitro and in vivo studies or between pre-clinical and clinical studies does not help in the current situation where a therapeutic agent for COVID-19 must be discovered in a short amount of time. Therefore, it is important to design in vivo or clinical studies after a thorough understanding of drug pharmacology and in-depth consideration of how to link in vitro antiviral activity and drug exposure at the putative target site of action. Fan et al. demonstrated that in vitro EC50/EC90 values for hydroxychloroquine should be compared to the in vivo free extracellular tissue concentration, which is similar to the free plasma hydroxychloroquine concentration [127]. Advances in cell modeling tools for biological research are expected to further enrich preclinical research design, and also help promote the development of new therapies [128].
When a specific infection enters a pandemic state, group immunization through vaccine, rather than quarantine, is the most effective countermeasure. As of October 29, 2020, 201 candidate vaccines against SARS-CoV-2 are being developed. Among them, 45 have entered clinical trials, and none has been approved for use yet [129].
Conclusion
In this summary report, we synthesized the results of previous studies on the treatment of SARS, MERS, and COVID-19. There was no therapeutic agent that consistently resulted in positive outcomes across SARS, MERS, and COVID-19. Remdesivir showed a therapeutic effect for COVID-19 in two RCTs involving the largest number of total participants (n = 1,461). Other therapies that showed an effect in at least two RCTs for COVID-19 were sofosbuvir/daclatasvir (n = 114), colchicine (n = 140), IFN-β1b (n = 193), and convalescent plasma therapy (n = 126). Further RCTs are required.
Abbreviations
ACEI: angiotensin-converting enzyme inhibitor; ACE2: angiotensin-converting enzyme 2; ARB: angiotensin receptor blocker; ARDS: acute respiratory distress syndrome; CI: confidence interval; COVID-19: coronavirus disease 2019; CRAC: calcium release-activated calcium; CT: computed tomography; EC50 : 50% maximal effective concentration; ExoN: exoribonuclease; FDA: Food and Drug Administration; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HR: hazard ratio; ICU: intensive care unit; IFN: interferon; IL: interleukin; IQR: interquartile range; MERS: Middle East respiratory syndrome; MV: mechanical ventilation; NEWS2: National Early Warning Score 2; OR: odds ratio; PCR: polymerase chain reaction; PICO: Participants, Interventions, Comparisons, and Outcomes; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT: randomized controlled trial; RdRp: RNA dependent RNA polymerase; rhG-CSF: recombinant human granulocyte colony-stimulating factor; RR: relative risk; SARS: severe acute respiratory syndrome; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SD: standard deviation; SI: selectivity index; SOFA: sequential organ failure assessment; WHO: World Health Organization.
Supplementary Material
Supplementary table S1.
Acknowledgements
We appreciate Kyungsoo Park, Professor of Department of Pharmacology, Yonsei University College of Medicine, Seoul, Republic of Korea, and Jae Yong Chung, Professor of Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine, Seoul, Republic of Korea. They contributed to this study by giving valuable advice on interpreting in vitro studies.
Author contributions
YJH and JIS conceived and designed the Review. YJH and JIS selected the articles, extracted and analyzed the data, and wrote the first draft of the manuscript. YJH, KHL, SY, SWN, SR, DS, JSK, JYL, JWY, JHL, AKo, SHH, ED, JR, LS, HO, RAG, AKr, ME, DK, SD, WK, LJ, HS, and JIS interpreted the data and contributed to the writing of the final version of the manuscript. All authors critically revised the manuscript and agreed with the results and conclusions of this Review.
Competing Interests
The authors have declared that no competing interest exists.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-62
3. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q. et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-5
4. Chan-Yeung M, Xu RH. SARS: epidemiology. Respirology. 2003;8(Suppl):S9-14
5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). https://www.who.int/emergencies/mers-cov/en/
6. Weissleder R, Lee H, Ko J, Pittet MJ. COVID-19 diagnostics in context. Sci Transl Med. 2020 12
7. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition). http://kjfy.meetingchina.org/msite/news/show/cn/3337.html
8. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343
9. Momattin H, Mohammed K, Zumla A, Memish ZA, Al-Tawfiq JA. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV)-possible lessons from a systematic review of SARS-CoV therapy. Int J Infect Dis. 2013;17:e792-8
10. Zhao R, Wang H, Wang X, Feng F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporos Int. 2017;28:1027-34
11. Chen Y, Guo JJ, Healy DP, Zhan S. Effect of integrated traditional Chinese medicine and western medicine on the treatment of severe acute respiratory syndrome: A meta-analysis. Pharm Pract (Granada). 2007;5:1-9
12. Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS. et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80-90
13. Momattin H, Al-Ali AY, Al-Tawfiq JA. A Systematic Review of therapeutic agents for the treatment of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Travel Med Infect Dis. 2019;30:9-18
14. Morra ME, Van Thanh L, Kamel MG, Ghazy AA, Altibi AMA, Dat LM. et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: A systematic review and meta-analysis. Rev Med Virol. 2018;28:e1977
15. Dawson P, Malik MR, Parvez F, Morse SS. What Have We Learned About Middle East Respiratory Syndrome Coronavirus Emergence in Humans? A Systematic Literature Review. Vector Borne Zoonotic Dis. 2019;19:174-92
16. Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol. 2020;38:10-8
17. Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020 https://doi.org/10.1002/jmv.25729
18. A Multi-centre, Double-blinded, Randomized, Placebo-controlled Trial on the Efficacy and Safety of Lopinavir / Ritonavir Plus Ribavirin in the Treatment of Severe Acute Respiratory Syndrome. https://ClinicalTrials.gov/show/NCT00578825
19. Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, AlJohani S, Al Harbi S. et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81
20. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC. et al. Remdesivir for the Treatment of Covid-19 - Preliminary Report. N Engl J Med. 2020
21. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-78
22. Misra S, Nath M, Hadda V, Vibha D. Efficacy of various treatment modalities for nCOV-2019: A systematic review and meta-analysis. Eur J Clin Invest. 2020 p: e13383
23. Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A. et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:1048-57
24. Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R. et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 https://doi.org/10.1056/NEJMoa2015301
25. Maffei D. Gilead Announces Results From Phase 3 Trial of Remdesivir in Patients with Moderate COVID-19. Available from: https://www.businesswire.com/news/home/20200601005310/en/. 2020
26. Jiang Y, Chen D, Cai D, Yi Y, Jiang S. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: A network meta-analysis. J Med Virol. 2020 https://doi.org/10.1002/jmv.26443
27. Sadeghi A, Ali Asgari A, Norouzi A, Kheiri Z, Anushirvani A, Montazeri M. et al. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. J Antimicrob Chemother. 2020 https://doi.org/10.1093/jac/dkaa334
28. Abbaspour Kasgari H, Moradi S, Shabani AM, Babamahmoodi F, Davoudi Badabi AR, Davoudi L. et al. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother. 2020 https://doi.org/10.1093/jac/dkaa332
29. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-71
30. Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY. et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786
31. Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN. et al. AVIFAVIR for Treatment of Patients with Moderate COVID-19: Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clin Infect Dis. 2020 https://doi.org/10.1093/cid/ciaa1176
32. Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M. et al. A prospective, randomized, open-label trial of early versus late favipiravir in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020 https://doi.org/10.1128/aac.01897-20
33. Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H. et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6:28
34. Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y. et al. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med (N Y). 2020. https://doi.org/10.1016/j.medj. 2020 04.001
35. Huang D, Yu H, Wang T, Yang H, Yao R, Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Med Virol. 2020 https://doi.org/10.1002/jmv.26256
36. Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS. et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252-6
37. Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MM. et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399-406
38. Sheahan TP, Sims AC, Leist SR, Schafer A, Won J, Brown AJ. et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222
39. Chan JF, Yao Y, Yeung ML, Deng W, Bao L, Jia L. et al. Treatment With Lopinavir/Ritonavir or Interferon-beta1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J Infect Dis. 2015;212:1904-13
40. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G. et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-99
41. Huang YQ, Tang SQ, Xu XL, Zeng YM, He XQ, Li Y. et al. No Statistically Apparent Difference in Antiviral Effectiveness Observed Among Ribavirin Plus Interferon-Alpha, Lopinavir/Ritonavir Plus Interferon-Alpha, and Ribavirin Plus Lopinavir/Ritonavir Plus Interferon-Alpha in Patients With Mild to Moderate Coronavirus Disease 2019: Results of a Randomized, Open-Labeled Prospective Study. Front Pharmacol. 2020;11:1071
42. Wang M, Wu T, Zuo Z, You Y, Yang X, Pan L. et al. Evaluation of current medical approaches for COVID-19: a systematic review and meta-analysis. BMJ Support Palliat Care. 2020 https://doi.org/10.1136/bmjspcare-2020-002554
43. Liu W, Zhou P, Chen K, Ye Z, Liu F, Li X. et al. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. CMAJ. 2020;192:E734-E44
44. Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY. et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695-704
45. Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L. et al. A Randomized, Open-label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, A Pilot Study. Adv Sci (Weinh). 2020;7:2001435
46. Wu X, Yu K, Wang Y, Xu W, Ma H, Hou Y. et al. Efficacy and safety of triazavirin therapy for coronavirus disease 2019: A pilot randomized controlled trial. Engineering (Beijing). 2020. https://doi.org/10.1016/j.eng. 2020 08.011
47. Chen J, Xia L, Liu L, Xu Q, Ling Y, Huang D. et al. Antiviral Activity and Safety of Darunavir/Cobicistat for the Treatment of COVID-19. Open Forum Infect Dis. 2020;7:ofaa241
48. Yamamoto N, Yang R, Yoshinaka Y, Amari S, Nakano T, Cinatl J. et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004;318:719-25
49. Musarrat F, Chouljenko V, Dahal A, Nabi R, Chouljenko T, Jois SD. et al. The anti-HIV Drug Nelfinavir Mesylate (Viracept) is a Potent Inhibitor of Cell Fusion Caused by the SARS-CoV-2 Spike (S) Glycoprotein Warranting further Evaluation as an Antiviral against COVID-19 infections. J Med Virol. 2020 https://doi.org/10.1002/jmv.25985
50. Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L. et al. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother. 2006;17:275-84
51. de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM. et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875-84
52. Cong Y, Hart BJ, Gross R, Zhou H, Frieman M, Bollinger L. et al. MERS-CoV pathogenesis and antiviral efficacy of licensed drugs in human monocyte-derived antigen-presenting cells. PLoS One. 2018;13:e0194868
53. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P. et al. In vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 https://doi.org/10.1093/cid/ciaa237
54. Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H. et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16
55. Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D. et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020. 2020 03.22.20040758
56. Kamran SM, Mirza ZeH, Naseem A, Saeed F, Azam R, Ullah N. et al. Clearing the fog: Is HCQ effective in reducing COVID-19 progression: A randomized controlled trial. medRxiv. 2020. 2020 07.30.20165365
57. Sekhavati E, Jafari F, SeyedAlinaghi S, Jamalimoghadamsiahkali S, Sadr S, Tabarestani M. et al. Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial. Int J Antimicrob Agents. 2020;56:106143
58. Furtado RHM, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR, Lapa MG. et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959-67
59. Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW. et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-9
60. Auyeung TW, Lee JS, Lai WK, Choi CH, Lee HK, Lee JS. et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98-102
61. Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA. et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197:757-67
62. Sung JJ, Wu A, Joynt GM, Yuen KY, Lee N, Chan PK. et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414-20
63. Ho JC, Ooi GC, Mok TY, Chan JW, Hung I, Lam B. et al. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med. 2003;168:1449-56
64. Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W. et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003;52:715-20
65. Yam LY, Lau AC, Lai FY, Shung E, Chan J, Wong V. et al. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54:28-39
66. Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S. et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020 https://doi.org/10.1183/13993003.02808-2020
67. Lu S, Zhou Q, Huang L, Shi Q, Zhao S, Wang Z. et al. Effectiveness and safety of glucocorticoids to treat COVID-19: a rapid review and meta-analysis. Ann Transl Med. 2020;8:627
68. Ye Z, Wang Y, Colunga-Lozano LE, Prasad M, Tangamornsuksan W, Rochwerg B. et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192:E756-E67
69. Sarkar S, Khanna P, Soni KD. Are the steroids a blanket solution for COVID-19? A systematic review and meta-analysis. J Med Virol. 2020 https://doi.org/10.1002/jmv.26483
70. Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P. et al. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw Open. 2020;3:e2013136
71. Lopes MIF, Bonjorno LP, Giannini MC, Amaral NB, Benatti MN, Rezek UC. et al. Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial. medRxiv. 2020. 2020 08.06.20169573
72. Duarte M, Pelorosso FG, Nicolosi L, Salgado MV, Vetulli H, Aquieri A. et al. Telmisartan for treatment of Covid-19 patients: an open randomized clinical trial. Preliminary report. medRxiv. 2020. 2020 08.04.20167205
73. Pranata R, Permana H, Huang I, Lim MA, Soetedjo NNM, Supriyadi R. et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:983-90
74. Flacco ME, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A. et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020;106:1519-24
75. Lemos ACB, do Espírito Santo DA, Salvetti MC, Gilio RN, Agra LB, Pazin-Filho A. et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb Res. 2020;196:359-66
76. Lu YF, Pan LY, Zhang WW, Cheng F, Hu SS, Zhang X. et al. A meta-analysis of the incidence of venous thromboembolic events and impact of anticoagulation on mortality in patients with COVID-19. Int J Infect Dis. 2020;100:34-41
77. Abdel-Maboud M, Menshawy A, Elgebaly A, Bahbah EI, El Ashal G, Negida A. Should we consider heparin prophylaxis in COVID-19 patients? a systematic review and meta-analysis. J Thromb Thrombolysis. 2020 p: 1-3
78. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R. et al. "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study". J Steroid Biochem Mol Biol. 2020;203:105751
79. Miller J, Bruen C, Schnaus M, Zhang J, Ali S, Lind A. et al. Auxora versus standard of care for the treatment of severe or critical COVID-19 pneumonia: results from a randomized controlled trial. Crit Care. 2020;24:502
80. Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol. 2013;87:12552-61
81. Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L. et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137-46 e3
82. Hu K, Wang M, Zhao Y, Zhang Y, Wang T, Zheng Z. et al. A Small-Scale Medication of Leflunomide as a Treatment of COVID-19 in an Open-Label Blank-Controlled Clinical Trial. Virol Sin. 2020 https://doi.org/10.1007/s12250-020-00258-7
83. Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L. et al. A Randomized Clinical Trial of the Efficacy and Safety of Interferon beta-1a in Treatment of Severe COVID-19. Antimicrob Agents Chemother. 2020 64
84. Rahmani H, Davoudi-Monfared E, Nourian A, Khalili H, Hajizadeh N, Jalalabadi NZ. et al. Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial. Int Immunopharmacol. 2020;88:106903
85. Fu W, Liu Y, Liu L, Hu H, Cheng X, Liu P. et al. An open-label, randomized trial of the combination of IFN-κ plus TFF2 with standard care in the treatment of patients with moderate COVID-19. EClinicalMedicine. 2020 p: 100547
86. Zheng F, Zhou Y, Zhou Z, Ye F, Huang B, Huang Y. et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: A randomized, open-label, parallel-group trial. Int J Infect Dis. 2020;99:84-91
87. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J. et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020; https://doi.org/10.1001/jama. 2020 10044
88. Avendano-Sola C, Ramos-Martinez A, Munez-Rubio E, Ruiz-Antoran B, Malo de Molina R, Torres F. et al. Convalescent Plasma for COVID-19: A multicenter, randomized clinical trial. medRxiv. 2020. 2020 08.26.20182444
89. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. et al. Convalescent plasma in the management of moderate COVID-19 in India: An open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial). medRxiv. 2020. 2020 09.03.20187252
90. Cheng LL, Guan WJ, Duan CY, Zhang NF, Lei CL, Hu Y. et al. Effect of Recombinant Human Granulocyte Colony-Stimulating Factor for Patients With Coronavirus Disease 2019 (COVID-19) and Lymphopenia: A Randomized Clinical Trial. JAMA Intern Med. 2020; https://doi.org/10.1001/jamainternmed. 2020 5503
91. Sakoulas G, Geriak M, Kullar R, Greenwood K, Habib M, Vyas A. et al. Intravenous Immunoglobulin (IVIG) Significantly Reduces Respiratory Morbidity in COVID-19 Pneumonia: A Prospective Randomized Trial. medRxiv. 2020. 2020 07.20.20157891
92. Vlaar APJ, de Bruin S, Busch M, Timmermans S, van Zeggeren IE, Koning R. et al. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020 https://doi.org/10.1001/10.1016/s2665-9913(20)30341-6
93. Cruz LR, Baladron I, Rittoles A, Diaz PA, Valenzuela C, Santana R. et al. Treatment with an Anti-CK2 Synthetic Peptide Improves Clinical Response in Covid-19 Patients with Pneumonia. A Randomized and Controlled Clinical Trial. medRxiv. 2020. 2020 09.03.20187112
94. Malgie J, Schoones JW, Pijls BG. Decreased mortality in COVID-19 patients treated with Tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin Infect Dis. 2020 https://doi.org/10.1093/cid/ciaa1445
95. Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V. et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381-5
96. Sacramento CQ, Fintelman-Rodrigues N, Temerozo JR, da Silva Gomes Dias S, Ferreira AC, Mattos M. et al. The in vitro antiviral activity of the anti-hepatitis C virus (HCV) drugs daclatasvir and sofosbuvir against SARS-CoV-2. bioRxiv. 2020. 2020 06.15.153411
97. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449-63
98. Blaising J, Polyak SJ, Pecheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84-94
99. Nukoolkarn V, Lee VS, Malaisree M, Aruksakulwong O, Hannongbua S. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J Theor Biol. 2008;254:861-7
100. Wang RR, Yang QH, Luo RH, Peng YM, Dai SX, Zhang XJ. et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One. 2014;9:e105617
101. Quiros Roldan E, Biasiotto G, Magro P, Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): A role for iron homeostasis? Pharmacol Res. 2020;158:104904
102. Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR. et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 https://doi.org/10.1056/NEJMoa2022926
103. Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M. et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3:e208857
104. Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ. et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio. 2020 1834
105. Bessiere F, Roccia H, Deliniere A, Charriere R, Chevalier P, Argaud L. et al. Assessment of QT Intervals in a Case Series of Patients With Coronavirus Disease 2019 (COVID-19) Infection Treated With Hydroxychloroquine Alone or in Combination With Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020; https://doi.org/10.1001/jamacardio. 2020 1787
106. World Health Organization. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19.
107. U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and.
108. Faust SN, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL. et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020 https://doi.org/10.1001/10.1056/NEJMoa2021436
109. Schlesinger N, Firestein BL, Brunetti L. Colchicine in COVID-19: an Old Drug, New Use. Curr Pharmacol Rep. 2020 p: 1-9
110. Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799-802
111. Imai Y, Kuba K, Penninger JM. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. 2008;93:543-8
112. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA. et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605-10
113. Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J. et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693-705
114. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B. et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875-9
115. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-7
116. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z. et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324-9
117. Wang T, Chen R, Liu C, Liang W, Guan W, Tang R. et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362-e3
118. Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090-9
119. Waldron RT, Chen Y, Pham H, Go A, Su HY, Hu C. et al. The Orai Ca(2+) channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J Physiol. 2019;597:3085-105
120. Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H. et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest. 2013;123:887-902
121. Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V. et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787-98
122. Lussana F, Rambaldi A. Inflammation and myeloproliferative neoplasms. J Autoimmun. 2017;85:58-63
123. Teschner S, Burst V. Leflunomide: a drug with a potential beyond rheumatology. Immunotherapy. 2010;2:637-50
124. Wang Q, Guo H, Li Y, Jian X, Hou X, Zhong N. et al. Efficacy and Safety of Leflunomide for Refractory COVID-19: An Open-label Controlled Study. medRxiv. 2020. 2020 05.29.20114223
125. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545-8
126. Liu X, Cao W, Li T. High-Dose Intravenous Immunoglobulins in the Treatment of Severe Acute Viral Pneumonia: The Known Mechanisms and Clinical Effects. Frontiers in Immunology. 2020 11
127. Fan J, Zhang X, Liu J, Yang Y, Zheng N, Liu Q. et al. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin Infect Dis. 2020 https://doi.org/10.1093/cid/ciaa623
128. Raimondi MT, Donnaloja F, Barzaghini B, Bocconi A, Conci C, Parodi V. et al. Bioengineering tools to speed up the discovery and preclinical testing of vaccines for SARS-CoV-2 and therapeutic agents for COVID-19. Theranostics. 2020;10:7034-52
129. World Health Organization. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
130. Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW. et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69-75
131. Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J Jr. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326:905-8
132. Tan EL, Ooi EE, Lin CY, Tan HC, Ling AE, Lim B. et al. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis. 2004;10:581-6
133. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045-6
134. Stroher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F. et al. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon- alpha. J Infect Dis. 2004;189:1164-7
135. Leung GM, Hedley AJ, Ho LM, Chau P, Wong IO, Thach TQ. et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141:662-73
136. Vu HT, Leitmeyer KC, Le DH, Miller MJ, Nguyen QH, Uyeki TM. et al. Clinical description of a completed outbreak of SARS in Vietnam, February-May 2003. Emerg Infect Dis. 2004;10:334-8
137. Hsu LY, Lee CC, Green JA, Ang B, Paton NI, Lee L. et al. Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg Infect Dis. 2003;9:713-7
138. Leong HN, Ang B, Earnest A, Teoh C, Xu W, Leo YS. Investigational use of ribavirin in the treatment of severe acute respiratory syndrome, Singapore, 2003. Trop Med Int Health. 2004;9:923-7
139. Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci Rep. 2013;3:1686
140. Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB. et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 9
141. Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X. et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018 9
142. Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773-9
143. de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T. et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117:6771-6
144. Shalhoub S, Al-Hameed F, Mandourah Y, Balkhy HH, Al-Omari A, Al Mekhlafi GA. et al. Critically ill healthcare workers with the middle east respiratory syndrome (MERS): A multicenter study. PLoS One. 2018;13:e0206831
145. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Treatment of SARS with human interferons. Lancet. 2003;362:293-4
146. Haagmans BL, Kuiken T, Martina BE, Fouchier RA, Rimmelzwaan GF, van Amerongen G. et al. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10:290-3
147. He R, Adonov A, Traykova-Adonova M, Cao J, Cutts T, Grudesky E. et al. Potent and selective inhibition of SARS coronavirus replication by aurintricarboxylic acid. Biochem Biophys Res Commun. 2004;320:1199-203
148. Zheng B, He ML, Wong KL, Lum CT, Poon LL, Peng Y. et al. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma). J Interferon Cytokine Res. 2004;24:388-90
149. Scagnolari C, Vicenzi E, Bellomi F, Stillitano MG, Pinna D, Poli G. et al. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther. 2004;9:1003-11
150. Spiegel M, Pichlmair A, Muhlberger E, Haller O, Weber F. The antiviral effect of interferon-beta against SARS-coronavirus is not mediated by MxA protein. J Clin Virol. 2004;30:211-3
151. Hensley LE, Fritz LE, Jahrling PB, Karp CL, Huggins JW, Geisbert TW. Interferon-beta 1a and SARS coronavirus replication. Emerg Infect Dis. 2004;10:317-9
152. Smits SL, de Lang A, van den Brand JM, Leijten LM, van IWF, Eijkemans MJ. et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6:e1000756
153. Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H. et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222-8
154. Lee PI, Hsueh PR. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect. 2020. https://doi.org/10.1016/j.jmii. 2020 02.001
155. Hart BJ, Dyall J, Postnikova E, Zhou H, Kindrachuk J, Johnson RF. et al. Interferon-beta and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol. 2014;95:571-7
156. Channappanavar R, Lu L, Xia S, Du L, Meyerholz DK, Perlman S. et al. Protective Effect of Intranasal Regimens Containing Peptidic Middle East Respiratory Syndrome Coronavirus Fusion Inhibitor Against MERS-CoV Infection. J Infect Dis. 2015;212:1894-903
157. Falzarano D, de Wit E, Rasmussen AL, Feldmann F, Okumura A, Scott DP. et al. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313-7
158. Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY. et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090-5
159. Habib AMG, Ali MAE, Zouaoui BR, Taha MAH, Mohammed BS, Saquib N. Clinical outcomes among hospital patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. BMC Infect Dis. 2019;19:870
160. Arabi YM, Shalhoub S, Mandourah Y, Al-Hameed F, Al-Omari A, Al Qasim E. et al. Ribavirin and Interferon Therapy for Critically Ill Patients With Middle East Respiratory Syndrome: A Multicenter Observational Study. Clin Infect Dis. 2020;70:1837-44
161. Garout MA, Jokhdar HAA, Aljahdali IA, Zein AR, Goweda RA, Hassan-Hussein A. Mortality rate of ICU patients with the Middle East Respiratory Syndrome - Coronavirus infection at King Fahad Hospital, Jeddah, Saudi Arabia. Cent Eur J Public Health. 2018;26:87-91
162. Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N. et al. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129-32
163. Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W. et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319-25
164. Sainz B Jr, Mossel EC, Peters CJ, Garry RF. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology. 2004;329:11-7
165. Arabi YM, Deeb AM, Al-Hameed F, Mandourah Y, Almekhlafi GA, Sindi AA. et al. Macrolides in critically ill patients with Middle East Respiratory Syndrome. Int J Infect Dis. 2019;81:184-90
166. Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG. et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69
167. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264-8
168. van Doremalen N, Falzarano D, Ying T, de Wit E, Bushmaker T, Feldmann F. et al. Efficacy of antibody-based therapies against Middle East respiratory syndrome coronavirus (MERS-CoV) in common marmosets. Antiviral Res. 2017;143:30-7
169. Ko JH, Seok H, Cho SY, Ha YE, Baek JY, Kim SH. et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617-22
170. Roberts A, Thomas WD, Guarner J, Lamirande EW, Babcock GJ, Greenough TC. et al. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis. 2006;193:685-92
171. Qiu H, Sun S, Xiao H, Feng J, Guo Y, Tai W. et al. Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal Middle East respiratory syndrome (MERS)-coronavirus infection. Antiviral Res. 2016;132:141-8
172. Li Y, Wan Y, Liu P, Zhao J, Lu G, Qi J. et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. 2015;25:1237-49
173. Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R Jr, Nunneley JW. et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76-84
174. Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J. et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885-93
175. Coleman CM, Sisk JM, Mingo RM, Nelson EA, White JM, Frieman MB. Abelson Kinase Inhibitors Are Potent Inhibitors of Severe Acute Respiratory Syndrome Coronavirus and Middle East Respiratory Syndrome Coronavirus Fusion. J Virol. 2016;90:8924-33
176. de Wilde AH, Raj VS, Oudshoorn D, Bestebroer TM, van Nieuwkoop S, Limpens R. et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-alpha treatment. J Gen Virol. 2013;94:1749-60
177. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787
178. Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S. et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343-55
179. Mitja O, Corbacho-Monne M, Ubals M, Tebe C, Penafiel J, Tobias A. et al. Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial. Clin Infect Dis. 2020 https://doi.org/10.1093/cid/ciaa1009
180. Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W. et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849
181. Abd-Elsalam S, Esmail ES, Khalaf M, Abdo EF, Medhat MA, Abd El Ghafar MS. et al. Hydroxychloroquine in the Treatment of COVID-19: A Multicenter Randomized Controlled Study. Am J Trop Med Hyg. 2020 https://doi.org/10.4269/ajtmh.20-0873
182. Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM. et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial. Ann Intern Med. 2020 https://doi.org/10.7326/M20-4207
183. Chen C-P, Lin Y-C, Chen T-C, Tseng T-Y, Wong H-L, Kuo C-Y. et al. A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19). medRxiv. 2020. 2020 07.08.20148841
184. Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN. et al. Convalescent Plasma for COVID-19. A randomized clinical trial. medRxiv. 2020. 2020 07.01.20139857
185. Balcells ME, Rojas L, Le Corre N, Martínez-Valdebenito C, Ceballos ME, Ferrés M. et al. Early Anti-SARS-CoV-2 Convalescent Plasma in Patients Admitted for COVID-19: A Randomized Phase II Clinical Trial. medRxiv. 2020. 2020 09.17.20196212
186. Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E. et al. Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS Med. 2020;17:e1003293
187. Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. 2020;17:141
188. Lou Y, Liu L, Qiu Y. Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: an Exploratory Randomized, Controlled Trial. medRxiv. 2020. 2020 04.29.20085761
189. Zhong H, Wang Y, Zhang ZL, Liu YX, Le KJ, Cui M. et al. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis. Pharmacol Res. 2020;157:104872
190. Elsawah HK, Elsokary MA, Elrazzaz MG, Elshafie AH. Hydroxychloroquine for treatment of nonsevere COVID-19 patients: Systematic review and meta-analysis of controlled clinical trials. J Med Virol. 2020 https://doi.org/10.1002/jmv.26442
191. Yang TH, Chou CY, Yang YF, Chien CS, Yarmishyn AA, Yang TY. et al. Systematic Review and Meta-analysis of the Effectiveness and Safety of Hydroxychloroquine in Treating COVID-19 Patients. J Chin Med Assoc. 2020 https://doi.org/10.1097/jcma.0000000000000425
192. Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L. et al. [A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215-9
193. Das RR, Jaiswal N, Dev N, Naik SS, Sankar J. Efficacy and Safety of Anti-malarial Drugs (Chloroquine and Hydroxy-Chloroquine) in Treatment of COVID-19 Infection: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2020;7:482
194. Thoguluva Chandrasekar V, Venkatesalu B, Patel HK, Spadaccini M, Manteuffel J, Ramesh M. Systematic review and meta-analysis of effectiveness of treatment options against SARS-CoV-2 infection. J Med Virol. 2020 https://doi.org/10.1002/jmv.26302
195. Zang Y, Han X, He M, Shi J, Li Y. Hydroxychloroquine use and progression or prognosis of COVID-19: a systematic review and meta-analysis. Naunyn Schmiedebergs Arch Pharmacol. 2020 p: 1-8
196. Putman M, Chock YPE, Tam H, Kim AHJ, Sattui SE, Berenbaum F. et al. Antirheumatic Disease Therapies for the Treatment of COVID-19: A Systematic Review and Meta-analysis. Arthritis Rheumatol. 2020 https://doi.org/10.1002/art.41469
197. Sarma P, Kaur H, Kumar H, Mahendru D, Avti P, Bhattacharyya A. et al. Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis. J Med Virol. 2020;92:776-85
198. Talaie H, Hosseini SM, Nazari M, Fakhri Y, Mousavizadeh A, Vatanpour H. et al. Is there any potential management against COVID-19? A systematic review and meta-analysis. Daru. 2020 p: 1-13
199. Ullah W, H MA, Roomi S, Sattar Y, Almas T, Narayana Gowda S. et al. Safety and Efficacy of Hydroxychloroquine in COVID-19: A Systematic Review and Meta-Analysis. J Clin Med Res. 2020;12:483-91
200. Singh AK, Singh A, Singh R, Misra A. "Hydroxychloroquine in patients with COVID-19: A Systematic Review and meta-analysis.". Diabetes Metab Syndr. 2020;14:589-96
201. Elavarasi A, Prasad M, Seth T, Sahoo RK, Madan K, Nischal N. et al. Chloroquine and Hydroxychloroquine for the Treatment of COVID-19: a Systematic Review and Meta-analysis. J Gen Intern Med. 2020 p: 1-7
202. Sarkar S, Soni KD, Khanna P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol. 2020 https://doi.org/10.1002/jmv.26408
203. Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103
Author contact
![]() Corresponding author: Dr. Jae Il Shin MD PhD, 50-1 Yonsei-ro, Seodaemun-gu, Department of Pediatrics, Yonsei University College of Medicine, Seoul 03722, Republic of Korea. Tel: 82-2-2228-2050, Fax: 82-2-393-9118, E-mail: shinjiac.
Corresponding author: Dr. Jae Il Shin MD PhD, 50-1 Yonsei-ro, Seodaemun-gu, Department of Pediatrics, Yonsei University College of Medicine, Seoul 03722, Republic of Korea. Tel: 82-2-2228-2050, Fax: 82-2-393-9118, E-mail: shinjiac.
 Global reach, higher impact
Global reach, higher impact