13.3
Impact Factor
Theranostics 2021; 11(6):2594-2611. doi:10.7150/thno.51648 This issue Cite
Research Paper
Group 2 innate lymphoid cells contribute to IL-33-mediated alleviation of cardiac fibrosis
1. Institute for Translational Research in Biomedicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
2. Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan.
3. Division of Cardiology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung City, Taiwan.
4. Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung City, Taiwan.
5. Division of Nephrology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung City, Taiwan.
#These authors contributed equally to this work.
Abstract
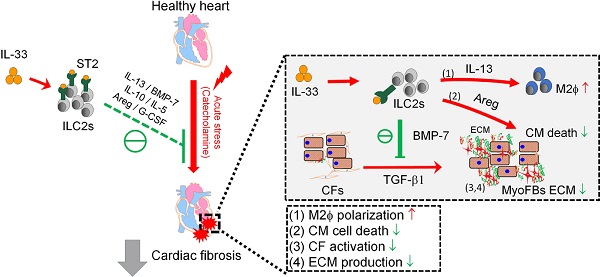
Rationale: The major cause of heart failure is myocardium death consequent to detrimental cardiac remodeling and fibrosis following myocardial infarction. The cardiac protective cytokine interleukin (IL)-33, which signals by ST2 receptor binding, is associated with group 2 innate lymphoid cell (ILC2) activation and regulates tissue homeostasis and repair following tissue injury in various tissues. However, the distribution and role of IL-33-responsive ILC2s in cardiac fibrosis remain unclear. In this study, we elucidated the roles of IL-33-responsive cardiac-resident ILC2s and IL-33-mediated immunomodulatory functions in cardiac fibrosis.
Methods: We examined the distribution of cardiac ILC2s by using flow cytometry. The roles of IL-33-mediated ILC2 expansion in cardiac fibrosis was evaluated in the mouse model of catecholamine-induced cardiac fibrosis. ILC-deficient Rag2‒/‒IL2Rγc‒/‒ mice were implemented to determine the contribution of endogenous ILC in the progression of cardiac fibrosis. Histopathological assessments, speckle tracking echocardiography, and transcriptome profile analysis were performed to determine the effects of IL-33-mediated cardiac protective functions.
Results: We identified the resident cardiac ILC2s, which share similar cell surface marker and transcriptional factor expression characteristics as peripheral blood and lung tissue ILC2s. IL-33 treatment induced ILC2 expansion via ST2. In vivo, ILC-deficient Rag2‒/‒IL2Rγc‒/‒ mice developed exacerbated cardiac fibrosis following catecholamine-induced stress cardiac injury. IL-33 treatment expanded cardiac ILC2s and revealed protective effects against cardiac tissue damage with reduced cardiomyocyte death, immune cell infiltration, tissue fibrosis, and improved myocardial function. Transcriptome analysis revealed that IL-33 attenuated extracellular matrix synthesis- and fibroblast activation-associated gene expressions. IL13-knockout or epidermal growth factor receptor (EGFR) inhibition abolished IL-33-mediated cardiac protective function, confirming IL-13 and EGFR signaling as crucial for IL-33-mediated cardioprotective responses. Moreover, ILC2-produced BMP-7 served as a novel anti-fibrotic factor to inhibit TGF-β1-induced cardiac fibroblast activation.
Conclusion: Our findings indicate the presence of IL-33-responsive ILC2s in cardiac tissue and that IL-33-mediated ILC2 expansion affords optimal cardioprotective function via ILC2-derived factors. IL-33-mediated immunomodulation is thus a promising strategy to promote tissue repair and alleviate cardiac fibrosis following acute cardiac injury.
Keywords: interleukin-33, cardiac fibrosis, ILC2, fibroblast activation, myocardial injury
 Global reach, higher impact
Global reach, higher impact